Originally developed in England in 1954, chlorhexidine first became available in the United States on September 17, 1976, under the brand name Hibiclens®.1-3 Chlorhexidine alone is insoluble in water, so it is typically combined with glucuronic acid to form a chlorhexidine gluconate (CHG) salt that is soluble in both water and alcohol.3,4
A membrane-active agent, Chlorhexidine is primarily used to reduce the number of bacteria found on the skin, and is recommended for both surgical hand and general skin antisepsis due to its persistent presence on the skin.3 Despite these generally accepted roles, actual evidence of benefit is conflicting and continued use of chlorhexidine seems largely unjustified.
Mechanism of action
Chlorhexidine is believed to exert its antimicrobial effects through three mechanisms. The cationic nature of chlorhexidine enables it to specifically target the negatively charged bacterial envelope. At low concentrations, chlorhexidine disrupts the lipid bilayer, inducing leakage of ionic potassium, inorganic phosphates, amino acids, and other cytoplasmic material.3-7 This effect is primarily bacteriostatic in nature and neutralization or removal of chlorhexidine may allow the cell to recover.4 As the concentration of chlorhexidine increases to bactericidal levels, membrane leakage decreases as the biguanide causes nucleic acids and proteins to precipitate and coagulate.3-7 Additionally, high concentrations of chlorhexidine are thought to inhibit membrane-bound adenosine triphosphatase (ATPase), thereby reducing the cell’s ability to produce useable energy.4,5 Cytoplasmic coagulation and inhibition of ATPase result in cell death.4
Efficacy
The in vitro antimicrobial effects of chlorhexidine exposure have been repeatedly demonstrated; however, concerns regarding insufficient neutralization and conflicting in vivo results have cast serious doubt on the practical value of this agent. Chlorhexidine acts far more slowly than alcohol-based antiseptics and appears to exert an immediate antimicrobial effect similar to non-antimicrobial soap, leaving its clinical value highly debatable. Despite the demonstrated superiority of alcohol-based antiseptics both in vivo and in clinical trials, chlorhexidine has remained in use due to its purportedly persistent antimicrobial activity following application. However, in vivo studies indicate similar long-term reductions in normal flora for both alcohol and chlorhexidine. In light of the available literature, the rationale for chlorhexidine-based antisepsis appears largely unfounded.
In vitro spectrum
Chlorhexidine is effective against most Gram-positive and Gram-negative bacteria, has some activity against enveloped viruses and fungi, but is not sporicidal and lacks substantial activity against mycobacteria and nonenveloped viruses.3-9 Gram-positive and Gram-negative bacteria are typically susceptible to the bacteriostatic effects of chlorhexidine at minimum inhibitory concentrations (MICs) of 1 µg/mL and 2-2.5 µg/mL, respectively.5,7 Bactericidal effects are generally observed at concentrations ≥ 20 µg/mL.5,7 In a test of 1,165 bacterial isolates, the majority of strains were inhibited by 12.5 µg/mL of chlorhexidine and 100 µg/mL was sufficient to inhibit all evaluated strains.4 Since typical in-use concentrations range from 0.5-4% (5,000-40,000 µg/mL), clinical levels of exposure should exceed most reported MICs by several orders of magnitude.3,4
In vivo results
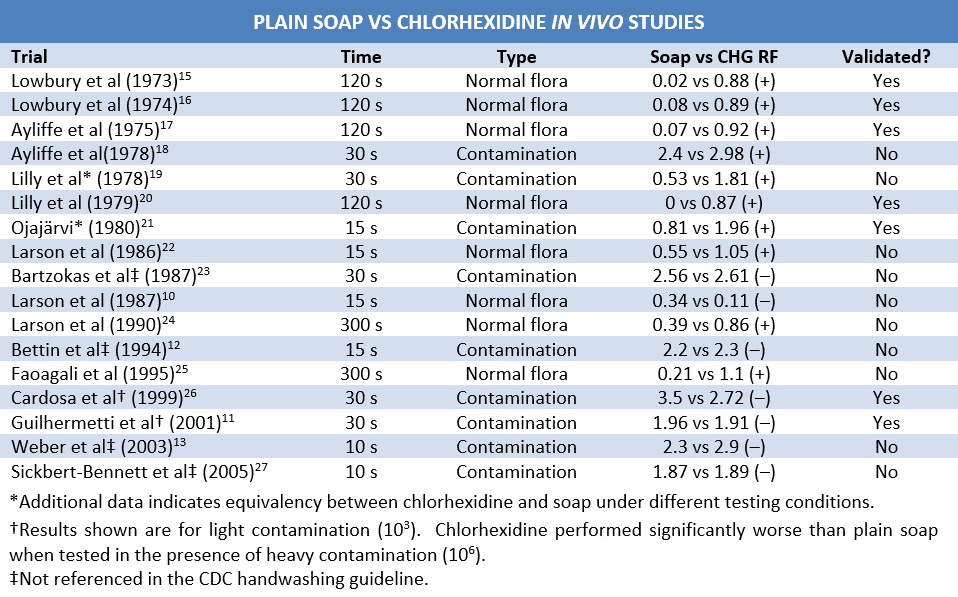
Despite the broad antimicrobial spectrum of chlorhexidine, in-use efficacy is at best marginally superior to plain soap. For example, a 15-second handwash with chlorhexidine showed no advantage over plain soap in reducing normal hand flora. Chlorhexidine did not demonstrate superiority until 15 washes had been performed, and 5 consecutive days of 15 hand washes resulted in a mean log10 reduction factor (RF) of 2.04 (vs. 0.59 for plain soap).10 In another study, fingertip-contamination with methicillin-resistant Staphylococcus aureus (MRSA) showed equivalent log10 RFs for chlorhexidine 4% and plain soap (1.91 vs. 1.96) following inoculation with 103 bacteria. Following inoculation with 106 bacteria, chlorhexidine was inferior to plain soap (1.37 vs. 1.77).11 Chlorhexidine was also inferior to plain soap in eliminating 106 Clostridium difficile (47% spores) from gloved hands (1.3 vs. 1.7) and 106 Bacillus anthracis surrogates (1.4 vs. 2.2).12,13 After a comprehensive literature review, Kampf et al (2004) estimated that chlorhexidine reduces transient flora by 2.1-3 log10 and resident flora by 0.35-1.75 log10 (vs. 0.5-3 and ≤ 0.4, respectively, for plain soap).7
Trends in the testing timeline provide further perspective. The vast majority of studies demonstrating the superiority of chlorhexidine over plain soap occurred around the time the biguanide was approved by the FDA, while most trials conducted in recent years have shown the two to be roughly equivalent. Assuming a lack of research bias, this can be largely attributed to differences in application time. Many early studies assessed the efficacy of chlorhexidine after 2 minutes of application. Since changes in time of exposure have shown to increase the RF of chlorhexidine from 1 log10 at 30 seconds to 1.74 log10 at 2 minutes and handwashing duration is typically 10 seconds or less, the results of these early studies cannot be assumed to apply to normal practice.3,14 In contrast to their predecessors, more recent studies utilizing relevant exposure times show a general lack of effect. These differences in methodology and results further undermine in vivo efficacy claims, particularly in settings that mimic clinical practice.
The Neutralization Controversy
Further complicating the efficacy debate, recent reevaluation of neutralizing agents used in chlorhexidine testing has cast considerable doubt on the validity of many in vitro and in vivo trials.28 Since residual chlorhexidine persists on surfaces after initial application, incomplete neutralization of chlorhexidine results in continued bacteriostatic or bactericidal effect in the sampling fluid, diluents and/or agar. This reportedly leads to a 2 log10 overestimation of reduction factor in vitro and in vivo.29-32 The discovery that commonly used neutralizing agent combinations (eg, 3.5% Tween 80, 0.5% lecithin, and 0.5% sodium thiosulfate) failed to inactivate chlorhexidine has generated significant concern regarding the dependability of the majority of chlorhexidine studies.28,32-34
The relevance of these issues is particularly evident when considering the in vivo chlorhexidine studies referenced by the CDC.3 While all of these studies utilized some sort of neutralizing agent, many failed to validate their neutralization process. The absence of demonstrable chlorhexidine neutralization renders the results of these trials unreliable. Since trials conducted without validation of neutralization can be assumed to err on the side of efficacy overestimation, it is reasonable to presume that trials which failed to show benefit in the absence of proven neutralizing agents would have failed to show benefit in their presence as well. Using this logic, of the dependable in vivo studies referenced by the CDC guideline, there is only a 5:3 superiority-to-inferiority ratio when comparing chlorhexidine with plain soap. Notably, the CDC guideline does not reference two articles with negative efficacy results authored prior to release of the guideline.12,28 Taking these trials into account, there is a 5:5 superiority-to-inferiority ratio of validated chlorhexidine vs. soap studies conducted prior to compilation of the CDC guidelines.
Clinical studies
Not surprisingly, the clinical benefits of chlorhexidine use are controversial.7,35 Positive efficacy studies are few and generally limited by poor compliance, inconsistent results, or comparison solely against povidone-iodine. One study showed similar efficacy between 2% chlorhexidine and 61% ethanol in a neonatal intensive care unit (ICU) with poor handwashing compliance over a 22-month period.36 Similarly, another ICU study showed improvement in total nosocomial infection rate with chlorhexidine vs. alcohol use; however, this was not associated with difference in length of stay, mortality, or total number of infected patients.37 Chlorhexidine 4% also demonstrated better general antibacterial activity than triclosan 1% (0.27 vs. 0.07 log10 RF) during a 16-month longitudinal study, though chlorhexidine was ineffective against MRSA.38 Additionally, multiple clinical trials have demonstrated the superiority of chlorhexidine over povidone-iodine; however, both biocides appear to be less effective than alcohol-based antiseptics.4,39-41
In contrast to these studies, chlorhexidine use has been frequently associated with a lack of clinical benefit. Despite its purported persistent antimicrobial activity, chlorhexidine was unable to maintain resident bacterial counts below baseline values during operations lasting ≥ 3 hours (4.5 preoperatively vs. 5.2 postoperatively).42 Frequent handwashing with chlorhexidine in a neonatal ICU was associated with a doubling in baseline bacterial colonization over the course of a month.43 Marena et al reported that chlorhexidine was significantly less effective than plain soap in reducing hand contamination in a 4-month crossover study of two surgical wards.44 The results of these and other studies leave the clinical value of chlorhexidine use highly debatable.45,46
Persistent activity
In spite of mediocre efficacy and dubious clinical benefit, chlorhexidine is still used in a variety of roles thanks to its non-volatility and ability to adsorb to surfaces. Five hours after application, 93% of radio-labeled chlorhexidine remains present on uncovered skin, which correlates well with claims of persistent antimicrobial effect for 5-6 hours. Twenty-four hours after application of a 5% tincture, concentrations of 12.2 µg/mL (~0.25%) remain on the skin.4 Chlorhexidine appears to adsorb less readily to inorganic surfaces—approximately 1% binds to glass.47 Overall, these results indicate the presence of residual chlorhexidine greatly outlasts the original application time, though the clinical relevance of this non-volatility has never been demonstrated.7
The CDC defines persistent activity as “prolonged or extended antimicrobial activity that prevents or inhibits the proliferation or survival of microorganisms after application of the product.”3 Multiple trials conducted in an effort to demonstrate the persistent activity of chlorhexidine have reported mixed results. These results are complicated by the synergistic effects of alcohol on residual chlorhexidine activity as well as a lack of neutralization validation. Combination with alcohol appears to enhance the antimicrobial effects of residual chlorhexidine, making it difficult to quantify the independent activity of either agent.48 Additionally, while the need for proper, verifiable neutralization appears to add weight to residual antimicrobial activity arguments, insufficient neutralization makes it difficult to tell whether changes in microbial count are due to the residual presence of chlorhexidine on the skin or the presence of chlorhexidine in the growth medium after sampling. This concept is corroborated by the results of an “ex-vivo” study, where the presence of chlorhexidine in solution resulted in a 3-4 log10 greater reduction of pathogenic bacteria than observed when chlorhexidine was applied to skin samples.49
Suppression of Normal Flora
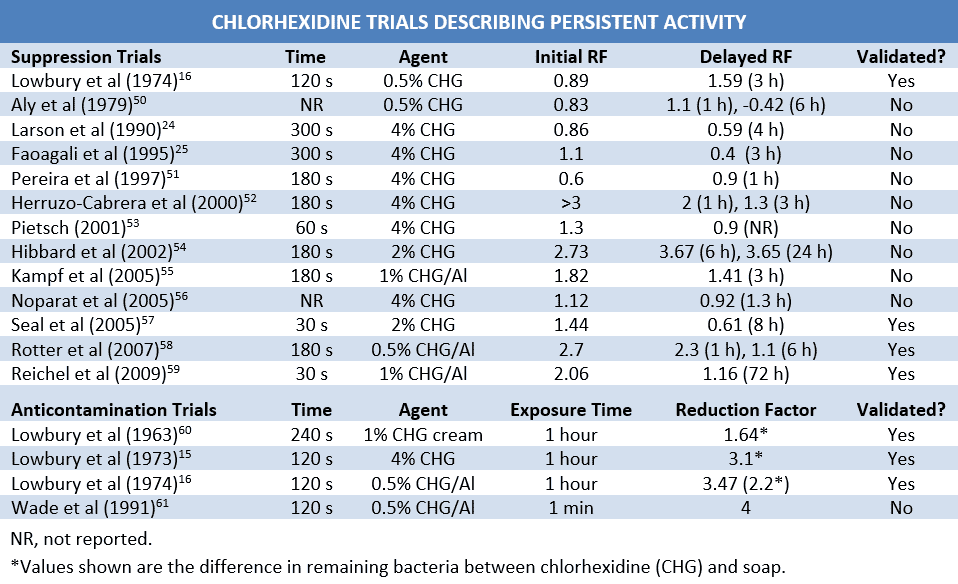
The CDC handwashing guideline cites eight studies as proof that chlorhexidine exhibits persistent antimicrobial activity.3 On close inspection, however, the majority of these articles provide little evidence to support this claim.28 One “study” is actually one of the first review articles to point out the need for proper chlorhexidine neutralization, arguing that the reported persistence of chlorhexidine may be due to its action in sampling fluids.29 A second article reports only the immediate effects of chlorhexidine.18 A third claims to demonstrate the superiority of 0.5% chlorhexidine over 70% isopropyl alcohol in reducing bacterial counts on the hands. However, despite multiple daily washings, chlorhexidine use was associated with increased baseline colonization increased (5.76 to 5.92 log10 CFUs) and 6-hour regrowth that was far more rapid than that observed with alcohol (see below).50

Other articles cited by the CDC are less contrary, but still inconclusive. In one, both chlorhexidine and alcohol—alone and in combination—were associated with an approximately 1 log10 larger reduction in normal flora after 3 hours of glove use than after initial administration, but there was no significant difference in RF between agents at any point.16 Another study reported slower normal flora recovery with 4% chlorhexidine than with a 70% ethyl alcohol and 0.5% chlorhexidine combination, however, overall bacterial reduction was greater with the combination product regardless of number of washings or the length of time after antisepsis.22 Lastly, Pereira et al compared various strengths of chlorhexidine and combinations with alcohol. Though all tested groups showed a greater reduction in CFUs 2 hours after initial administration than at administration, post-exposure CFU counts were higher following a second washing, suggesting swift bacterial recovery between treatments.51
Many other trials have been conducted in an effort to quantify the persistent activity of chlorhexidine. The majority of these have found chlorhexidine alone or in combination with alcohol to be approximately equal to or slightly inferior to alcohol-based hand-rubs in suppressing the normal flora.16,23,53-58 Faoagali et al reported a mild persistent effect on the first day of administration, though this disappeared after 5 days of twice-daily antisepsis.25 Results from another study indicated that delay of 72-hour regrowth of the normal flora on the upper arms and back was greater for the combination of chlorhexidine and n-propanol than for n-propanol alone by approximately 1 log10. Baseline CFU counts, however, were far lower than those typically observed on hands (~2.5 vs. ~ 6 log10), and delay of regrowth may have been due in part to virtual elimination of the natural flora during initial antisepsis.59 These results, along with those previously discussed, indicate that while chlorhexidine may mildly suppress the normal flora over time, the effects are controversial and of limited clinical relevance.
Contamination prevention
Bacterial contamination after antisepsis can also be used to assess the persistent activity of a biocide. Studies evaluating the ability of chlorhexidine to prevent new bacterial adherence and survival are limited, though their results are favorable. In 3 separate studies, Lowbury et al inoculated hands immediately following antisepsis with 20-50 µL suspensions of Staphylococcus aureus or Escherichia coli. The skin was allowed to dry for 2 minutes, rinsed and dried on a sterile towel, and then sampled 1 hour later.15,16,60 The quantity of bacteria administered was not reported in two of these trials, which reported only the number of viable bacteria per mL after sampling for chlorhexidine and plain soap. Both trials showed a substantially greater decrease in surviving bacteria following chlorhexidine use.15,60 Initial inoculum counts were included in the third trial, which compared 0.5% chlorhexidine in 95% ethyl alcohol with 70% ethyl alcohol. Alcoholic chlorhexidine demonstrated a much greater ability to prevent artificial S. aureus contamination than alcohol alone (3.47 vs -0.01) after inoculation with 4.7 log10 of bacteria. Intriguingly, while Lowbury also tested both biocides against E. coli, they did not report these results due to an unexpected residual effect in the alcohol group.16 Unfortunately, prolonged application time and immediate administration of a bacterial suspension make the results difficult to extrapolate to clinical practice.
Resistance
Given the already low efficacy profile of chlorhexidine, increasing reports of bacterial resistance are particularly concerning. Most Gram-positive and many Gram-negative bacteria lack significant resistance to chlorhexidine, though increases in MIC have been noted in some strains of MRSA, Escherichia coli O157, Salmonella enterica and Acinetobacter baumannii.62-68 In contrast, effective resistance to 0.5% chlorhexidine is often observed in clinical strains of Providencia stuartii (83.3%), Pseudomonas spp. (45.7%), Proteus spp. (38.7%) and Klebsiella spp. (1.8%).62 While the link between biocide resistance and antibiotic resistance has not yet been completely elucidated, many strains with significant chlorhexidine resistance also exhibit broad antibiotic resistance.62-64,69-71 Fortunately, exposure to residual chlorhexidine does not seem to engender meaningful resistance.47,72,73 However, regardless of reported planktonic MICs—most of which are irrelevant at in-use concentrations—chlorhexidine resistance and subsequent pathogenicity appear to be primarily due to the formation of biofilms.
Biofilms
Bacterial biofilms have high chlorhexidine resistance that allows constituent members to survive in concentrations 100s-1000s of times greater than the MICs reported for their planktonic counterparts.74-78 Biofilms form readily on almost any surface and are associated with an estimated 65% of nosocomial infections.77-79 Bacteria exhibit varying propensities for biofilm formation, which correspondingly affects their resistance to chlorhexidine. For example, in vitro biofilm studies demonstrated that 1% chlorhexidine eliminated 1.3 log10 of S. aureus and 1.6 log10 of Salmonella spp. after 5 minutes of exposure and only 0.22 log10 of P. aeruginosa after 30 minutes of exposure time.80,81 Clearly, biofilm-conferred resistance permits microorganisms to survive for prolonged periods in the presence of chlorhexidine.
Clinically speaking, biofilm formation not only enables biocide survival on fomites and environmental surfaces but may also directly facilitate infection outbreaks through contaminated chlorhexidine solutions. 82 Serratia marcescens biofilms are able to survive in chlorhexidine for years and can be cultured from solutions containing 2% chlorhexidine.83 Contamination of chlorhexidine solutions by Serratia marcescens and Pseudomonas aeruginosa has been repeatedly reported and linked to outbreaks of infection.68,82,83 Ironically, biofilm contamination is such an omnipresent infection risk that it is recommended that stock solutions of chlorhexidine be periodically disinfected with alcohol.68,82
Hindering factors
Putting clinical efficacy aside, a variety of substances undermine the killing ability of chlorhexidine. As a cationic molecule, chlorhexidine is inactivated natural soaps or other anionic compounds, non-ionic surfactants, cork, and hand creams containing anionic emulsifying agents.2-8,84 The activity of chlorhexidine is reduced in the presence of organic material (eg, blood, sputum), though it remains more far more effective than other cationic compounds.2,3,8,85 At concentrations ≥ 0.05%, chlorhexidine precipitates from solution as an insoluble salt when combined with borates, bicarbonates, carbonates, citrates, nitrates, phosphates, sulfates, and most dyes.3 Additionally, chlorhexidine may also be neutralized by hard water.3
Toxicity
Chlorhexidine use is typically well-tolerated and has a good safety record, though reports of skin irritation with chlorhexidine use range as high as 90% and can lead to poor use rates.3,4,8,9,55,86 Since chlorhexidine is not absorbed through the skin, systemic exposure following topical administration is extremely unlikely and has not been reported.4,87,88 There are 5 documented cases of acute toxicity following ingestion of high doses of chlorhexidine that manifested as gastrointestinal erosion, gastritis, and/or acute liver toxicity.4 Ocular exposure to preparations containing > 1% chlorhexidine can cause conjunctivitis and/or severe corneal damage and care should be taken to avoid touching the eyes after application.3,4,89 Sensorineural deafness has been reported in patients who received 0.05% chlorhexidine in 70% alcohol for perioperative disinfection of the ear. Additionally, allergic reactions can occur, particularly when chlorhexidine is used in the genital area or on neonates, and this hypersensitivity has lead to non-fatal anaphylaxis in several cases.4 However, considering the widespread, decades-long use of chlorhexidine, the incidence of toxic effects is remarkably low and chlorhexidine appears to be safe for general use.
Environmental effects
While chlorhexidine has an excellent topical safety profile, widespread use of the biguanide has raised concerns regarding the role of chlorhexidine in the environment.90 Chlorhexidine accumulates in the environment and may reach concentrations as high as 10.3 µg/mL (0.001%) in domestic wastewater.90,91 Concentrations of 10 µg/mL have been shown to have effects on algal and cyanobacterial biofilm, with bacterial biofilms beginning to change at concentrations of 100 µg/mL. The ultimate effects of this perturbation are unknown, but since biofilms form the foundation of the food chain, changes at this level may have broad consequences. Additionally, microbes appear to lack the ability to render chlorhexidine inert, which may result in progressive environmental accumulation.90 Since the EPA has determined that chlorhexidine is not only toxic to microbes but also slightly toxic to birds, moderately to highly toxic to fish and very highly toxic to invertebrates, continued use at current rates may lead to rising levels of environmental exposure with far-reaching ecological effects.89-91
Uses
Orally, chlorhexidine gluconate is used to reduce flora in the mouth for a variety of therapeutic and prophylactic purposes. Chlorhexidine binds strongly to negatively charged oral surfaces (eg, hydroxyapatite on tooth enamel, pellicle on the tooth surface), and is reported to exert an inhibitory effect on oral flora anywhere from 2 days to 12 weeks.92,93 The true residual oral activity of chlorhexidine is somewhat debatable; however, since biofilms of resident oral bacteria such as Streptococcus mutans demonstrate marked resistance to 0.2% chlorhexidine and may not be accounted for during the spit tests used to determine persistent activity.94 Despite these conflicting reports, chlorhexidine is used clinically to treat gingivitis (0.12%) and periodontitis (biodegradable subgingival pellets). Additionally, chlorhexidine mouthwash is used to prevent dental carries and plaque when normal oral hygiene is impossible, to reduce the incidence of mucositis and other oral complications in immunocompromised patients, and to decrease the risk of nosocomial respiratory tract infections in critically ill patients. Chlorhexidine appears to be highly effective in preventing respiratory tract infections, but efficacy data for other prophylactic oral uses is poor.92
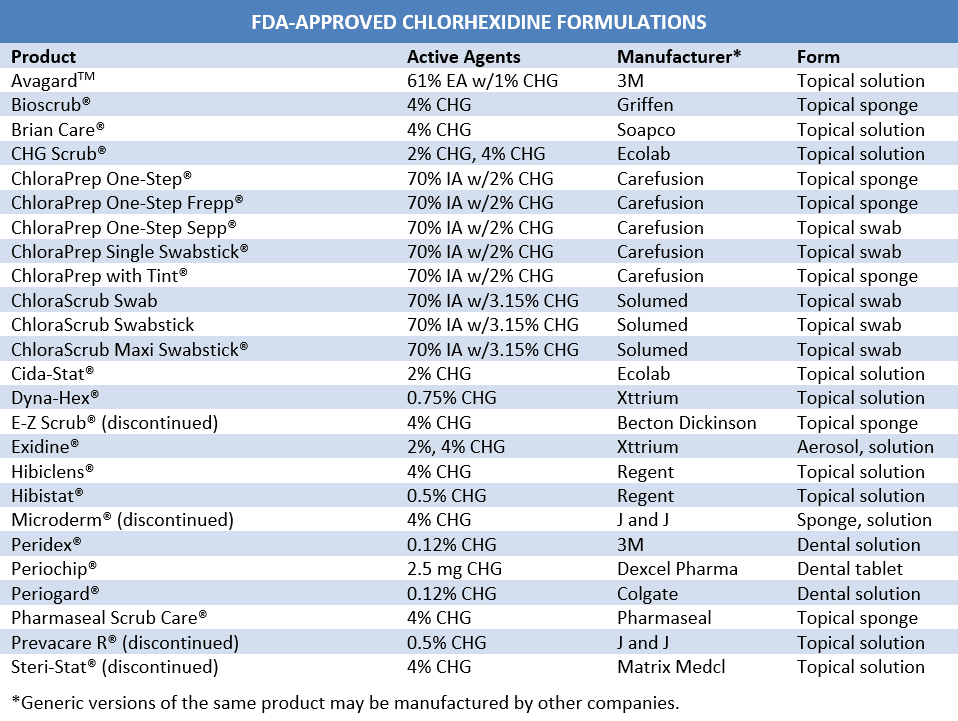
Alternative formulations may offer ways to circumvent some of the limitations of chlorhexidine. Catheters impregnated with chlorhexidine and silver sulfadiazine have been shown to reduce catheter-related bloodstream infections by approximately 60%, though efficacy has been somewhat inconsistent.95-97 Additionally, while still approved in the United States, these catheters have been discontinued in Japan due to severe anaphylactic reactions associated with use.97-99 Another formulation currently under development utilizes chlorhexidine-loaded nanocapsules to achieve a slow-release, sustained antimicrobial effect. Nanochlorex® appears to have a similar immediate effect to 62% propanol on resident bacteria and a dramatically superior effect (5.5 log10 vs 1 log10) on the survival of transient Staphylococcus epidermidis. However, this persistent activity disappears after 4 hourly inoculations with 1 mL of 107 bacteria.100,101 These preparations may provide more clinical benefit than currently available forms.
Conclusion
Chlorhexidine’s persistent presence in the market appears to be as unwarranted as claims of chlorhexidine’s persistent antimicrobial activity. While the membrane permeabilizing abilities of chlorhexidine give it a good in vitro profile, in vivo tests of immediate effect demonstrate a mild, equivocal superiority to plain soap, particularly at in-use application times. The vaunted residual presence of chlorhexidine also appears to be largely irrelevant, since numerous studies have shown an overall equivalence or inferiority to alcohol in long-term reduction of normal flora. Chlorhexidine may have some role in preventing exogenous recontamination, but this is undermined by poor clinical efficacy data, microbial resistance, limited effect against biofilms, skin dehydration and environmental accumulation. Despite this dearth of proven benefit, since the biguanide appears to provide the best persistent activity of currently available agents, chlorhexidine will likely remain in general use until the advent of a practical alternative.
References
- Davies GE, Francis J, Martin AR, Rose FL, Swain G. 1:6-Di-4’-chlorophenyldiguanidohexane (“Hibitane”). Laboratory investigation of a new antibacterial agent of high potency. Br J Pharmacol. 1954;9:192-6.
- Drugs@FDA. Hibiclens. Silver Spring (MD):U.S. Food and Drug Administration. September 17, 1976; [about 2 screens]; Available from http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=
DrugDetails. - Boyce JM, Pittet D; Healthcare Infection Control Practices Advisory Committee; HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hygiene Task Force. Am J Infect Control. 2002 Dec;30(8):S1-46.
- Chlorhexidine Gluconate Misc. In: McEvoy GK, Snow EK, Miller J, Kester L, Welsh OH Jr, Heydorn JD, Le T, Mendham NA, O’Rourke A, Braun S, Grande KJ, Litvak K, editors. AHFS Drug Information [Internet]. Bethesda: American Society of Health-System Pharmacists, Inc. c2010; [cited 2010 Oct 9]; [about 4 screens]. Available from http://www.medscape.com/druginfo/monograph?cid=med&drugid=4264&drugname=Chlorhexidine+
Gluconate+Misc&monotype=monograph&secid=4. Subscription required to view. - Russell AD, Chopra I. Understanding Antibacterial Action and Resistance, 2nd ed. Hertfordshire, England: Ellis Horwood. 1996. 292 p.
- Rotter ML. Hand washing and hand disinfection. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control, 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins. 2004:1727-46.
- Kampf G, Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin Microbiol Rev. 2004 Oct;17(4):863-93.
- Damani NN. Manual of Infection Control Procedures, 2nd ed. London: Greenwich Medical Media Limited. 2003. 333 p.
- Bradley C. Physical and chemical disinfection. In: Ayliffe GAJ, Fraise AP, Geddes AM, Mitchell K, editors. Conrtol of Hospital Infection: A Practical Handbook. New York: Oxford University Press, Inc. 2000:75-91.
- Larson EL, Laughon BE. Comparison of four antiseptic products containing chlorhexidine gluconate. Antimicrob Agents Chemother. 1987 Oct;31(10):1572-4.
- Guilhermetti M, Hernandes SED, Fukushigue Y, Garcia LB, Cardoso CL. Effectiveness of hand-cleansing agents for removing methicillin-resistant Staphylococcus aureus from contaminated hands. Infect Control Hosp Epidemiol. 2001 Feb;22(2):105-8.
- Bettin K, Clabots C, Mathie P, Willard K, Gerding DN. Effectiveness of liquid soap vs. chlorhexidine gluconate for the removal of Clostridium difficile from bare hands and gloved hands. Infect Control Hosp Epidemiol. 1994 Nov;15(11):697-702.
- Weber DJ, Sickbert-Bennett E, Gergen MF, Rutala WA. Efficacy of selected hand hygiene agents used to remove Bacillus atrophaeus (a surrogate of Bacillus anthracis) from contaminated hands. JAMA. 2003 Mar 12;289(10):1274-7.
- Lowbury EJL, Lilly HA, Bull JP. Methods for disinfection of hands and operation sites. Br Med J. 1964 Aug 29;2:531-6.
- Lowbury EJL, Lilly HA. Use of 4% chlorhexidine detergent solution (Hibiscrub) and other methods of disinfection. Br Med J. 1973;1:510-5.
- Lowbury EJL, Lilly HA, Ayliffe GAJ. Preoperative disinfection of surgeons’ hands: use of alcoholic solutions and effects of gloves on skin flora. Br Med J. 1974;4:369-72.
- Ayliffe GAJ, Babb JR, Bridges K, Lilly HA, Lowbury EJL, Varney J, Wilkins MD. Comparison of two methods for assessing the removal of total organisms and pathogens from the skin. J Hyg (Lond). 1975;75:259-274.
- Ayliffe GAJ, Babb JR, Quoraishi AH. A test for ‘hygienic’ hand disinfection. J Clin Pathol. 1978;31:923-8.
- Lilly HA, Lowbury EJL. Transient skin flora: their removal by cleansing or disinfection in relation to their mode of deposition. J Clin Path 1978;31:919–22.
- Lilly HA, Lowbury EJL, Wilkins MD. Detergents compared with each other and with antiseptics as skin ‘degerming’ agents. J Hyg (Lond). 1979;82:89-93.
- Ojajärvi J. Effectiveness of hand washing and disinfection methods in removing transient bacteria after patient nursing. J Hyg (Lond). 1980 Oct;85(2):193-203.
- Larson EL, Eke PI, Laughon BE. Efficacy of alcohol-based hand rinses under frequent-use conditions. Antimicrob Agents Chemother. 1986 Oct;30(4):542-4.
- Bartzokas CA, Corkill JE, Makin T. Evaluation of the skin disinfecting activity and cumulative effect of chlorhexidine and triclosan handwash preparations on hand artificially contaminated with Serratia marcescens. Infect Control. 1987;8(4):163-7.
- Larson EL, Butz AM, Gullette DL, Laughon BA. Alcohol for surgical scrubbing? 1990 Mar;11(3):139-43.
- Faoagali J, Fong J, George N, Mahoney P, O’Rouke V. Comparison of the immediate, residual, and cumulative antibacterial effects of Novaderm R, Novascrub R, Betadine Surgical Scrub, Hibiclens, and liquid soap. Am J Infect Control. 1995;23:337-43.
- Cardoso CL, Pereira HH, Zequim JC, Guilhermetti M. Effectiveness of hand-cleansing agents for removing Acinetobacter baumannii strain from contaminated hands. Am J Infect Control. 1999;27:327-31.
- Sickbert-Bennett EE, Weber DJ, Gergen-Teague MF, Sobsey MD, Samsa GP, Rutala WA. Comparative efficacy of hand hygiene agents in the reduction of bacteria and viruses. Am J Infect Control. 2005;33:67-77.
- Kampf G. What is left to justify the use of chlorhexidine in hand hygiene? J Hosp Infect. 2008 Oct;70 Suppl 1:27-34.
- Rotter ML. Hygienic hand disinfection. Infect Control. 1984;5:18-22.
- Cousido MC, Tomás M, Tomás I, Limeres J, García-Caballero L, Diz P. Effect of a neutralizing agent on the evaluation of the antimicrobial activity of chlorhexidine on the bacterial salivary flora. Arch Oral Biol. 2008;53:981-4.
- Sheikh W. Development and validation of a neutralizer system for in vitro evaluation of some antiseptics. Antimicrob Agents Chemother. 1981 Mar;19(3):429-34.
- Kampf G, Shaffer M, Hunte C. Insufficient neutralization in testing a chlorhexidine-containing ethanol-based hand rub can result in false positive efficacy assessment. BMC Infect Dis. 2005 Jun 20;5(1):48.
- Shimizu M, Okuzumi K, Yoneyama A, Kunisada T, Araake M, Ogawa H, Kimura S. In vitro antiseptic susceptibility of clinical isolates from nosocomial infections. Dermatology. 2002;204 Suppl 1:21-7.
- Reichel M, Heisig P, Kampf G. Pitfalls in efficacy testing – how important is the validation of neutralization of chlorhexidine digluconate? Ann Clin Microbiol Antimicrob. 2008;7:20.
- Kampf G. Effect of chlorhexidine probably overestimated because of lack of neutralization after sampling. Infect Control Hosp Epidemiol. 2009 Aug;30(8):811-2.
- Larson EL, Cimiotti J, Haas J, Parides M, Nesin M, Della-Latta P, Saiman L. Effect of antiseptic handwashing alcohol sanitizer on health care-associated infections in neonatal intensive care units. Arch Pediatr Adolesc Med. 2005;159:377-83.
- Doebbling BN, Stanley GL, Sheetz CT, Pfaller MA, Houston AK, Annis L, Li N, Wenzel RP. Comparative efficacy of alternative hand-washing agents in reducing nosocomial infections in intensive care units. N Engl J Med. 1992 Jul 9;327:88-93.
- Faoagali JL, George N, Fong J, Davy J, Dowser M. Comparison of the antibacterial efficacy of 4% chlorhexidine gluconate and 1% triclosan handwash products in an acute clinical ward. Am J Infect Control. 1999;27:320-6.
- Tanner J, Swarbrook S, Stuart J. Surgical hand antisepsis to reduce surgical infection. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD004288.
- Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Inter Med. 2002;136(11):792-801.
- Malani A, Trimble K, Parekh V, Chenoweth C, Kaufman S, Saint S. Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination. Infect Control Hosp Epidemiol. 2007 Jul;28(7):892-5.
- Bryce EA, Spence D, Roberts FJ. An in-use evaluation of an alcohol-based pre-surgical hand disinfectant. Infect Control Hosp Epidemiol. 2001 Oct;22(10):635-9.
- Ojajärvi J, Mäkelä P, Rantasalo I. Failure of hand disinfection with frequent hand washing: a need for prolonged field studies. J Hyg (Lond). 1977;79:107-19.
- Marena C, Lodola L, Zecca M, Bulgheroni A, Carretto E, Maserati X, Zambianchi L. Assessment of handwashing practices with chemical and microbiological methods: preliminary results from a prospective crossover study. Am J Infect Control. 2002;30:334-340.
- Johnson PDR, Martin R, Burrell LJ, Grabsch EA, Kirsa SW, O’Keefe J, Mayall BC, Edmonds D, Barr W, Bolger C, Naidoo H, Grayson ML. Efficacy of an alcohol/chlorhexidine hand hygiene program in a hospital with high rates of methicillin-resistant Staphylococcus aureus (MRSA) infection. MJA. 2005;183:509-14.
- Lowbury EJL, Lilly HA. Disinfection of the hands of surgeons and nurses. Br Med J. 1960 May 14;1:1445-49.
- Thomas L, Russell AD, Maillard JY. Antimicrobial activity of chlorhexidine diacetate and benzalkonium chloride against Pseudomonas aeruginosa and its response to biocide residues. J Appl Microbiol. 2005;98:533-43.
- Jarvis JD, Wynne CD, Enwright L, Williams JD. Handwashing and antiseptic-containing soaps in hospital. J Clin Pathol. 1979;32:732-7.
- Messager S, Goddard PA, Dettmar PW, Maillard JY. Determination of the antibacterial efficacy of several antiseptics tested on skin by an ‘ex-vivo’ test. J Med Microbiol. 2001;50:284-92.
- Aly R, Maibach HI. Comparative study on the antimicrobial effect of 0.5% chlorhexidine gluconate and 70% isopropyl alcohol on the normal flora of hands. Appl Environ Microbiol. 1979;37:610–613.
- Pereira LJ, Lee GM, Wade KJ. An evaluation of five protocols for surgical handwashing in relation to skin condition and microbial counts. J Hosp Infect. 1997;36:49-65.
- Herruzo-Cabrera R, Vizcaino-Alcaide MJ, Fdez-Aciñero. Usefulness of an alcoholic solution of N-duopropenide for the surgical antisepsis of the hands compared with handwashing with iodine-povidone and chlorhexidine: clinical essay. J Surg Res. 2000;94:6-12
- Peitsch H. Hand antiseptics: rubs versus scrubs, alcoholic solutions versus alcoholic gels. J Hosp Infect. 2001;48 Suppl A:S33-6.
- Hibbard JS, Mulberry GK, Brady AR. A clinical study comparing the skin antisepsis and safety of ChloraPrep, 70% isopropyl alcohol, and 2% aqueous chlorhexidine. J Infus Nurs. 2002 Jul-Aug;25(4):244-9.
- Kampf G, Oestermeyer C. Efficacy of two distinct ethanol-based hand rubs for surgical hand disinfection – a controlled trial according to prEN 12791. BMC Infectious Dis. 2005 Mar 22;5(1):17.
- Noparat W, Siripanichakom K, Tribuddharat C, Danchaivijitr S. Persistence of antimicrobial effect of antiseptics in surgical regimens. J Med Assoc Thai. 2005 Dec;88 Suppl 10:S177-82.
- Seal LA, Rizer RL, Maas-Irslinger R. A unique water optional health care personnel handwash provides antimicrobial perisistence and residual effects while decreasing the need for additional products. Am J Infect Control. 2005;33:207-16.
- Rotter ML, Kampf G, Suchomel M, Kundi M. Population kinetics of the skin flora on gloved hands following surgical hand disinfection with 3 propanol-based hand rubs: a prospective, randomized, double-blind trial. Infect Control Hosp Epidemiol. 2007 Mar;28(3):346-50.
- Reichel M, Heisig P, Kohlmann T, Kampf G. Alcohols for skin antisepsis at clinically relevant skin sites. Antimicrob Agents Chemother. 2009 Nov;53(11):4778-82.
- Lowbury EJL, Lilly HA, Bull JP. Disinfection of hands: removal of transient microorganisms. Br Med J. 1964 Jul 25;2:230-3.
- Wade JJ, Casewell MW. The evaluation of residual antimicrobial activity on hands and its clinical relevance. J Hosp Infect. 1991 Jun;18 Suppl B;23-8.
- Stickler DJ, Thomas B. Antiseptic and antibiotic resistance in Gram-negative bacteria causing urinary tract infection. J Clin Pathol. 1980;33:288-96.
- Brooks SE, Walczak MA, Hameed R, Coonan P. Chlorhexidine resistance in antibiotic-resistant bacteria isolated from the surfaces of dispensers of soap containing chlorhexidine. Infect Control Hosp Epidemiol. 2002 Nov;23(11):692-5.
- Kõljalg S, Naaber P, Mikelsaar M. Antibiotic resistance as an indicator of bacterial chlorhexidine susceptibility. J Hosp Infect. 2002;51:106-13.
- Stickler DJ. Susceptibility of antibiotic-resistant Gram-negative bacteria to biocides: a perspective from the study of catheter biofilms. J Appl Microbiol. 2002;92 Suppl:163S-170S.
- Cookson BD, Bolton MC, Platt JH. Chlorhexidine resistance in methicillin-resistant Staphylococcus aureus or just an elevated MIC? An in vitro and in vivo Antimicrob Agents Chemother. 1991 Oct;35(10):1997-2002.
- Braoudaki M, Hilton AC. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli 0157 and cross-resistance to antimicrobial agents. J Clin Microbiol. 2004 Jan;42(1):73-8.
- Burdon DW, Whitby JL. Contamination of hospital disinfectants with Pseudomonas Br Med J. 1967 Apr 15;2:153-5.
- Bloomfield SF. Significance of biocide usage and antimicrobial resistance in domiciliary environments. J Appl Microbiol Symp Suppl. 2002;92:144S-157S.
- Fraise AP. Susceptibility of antibiotic-resistant cocci to biocides. J Appl Microbiol Symp Suppl. 2002;92:158S-162S.
- Russell AD. Mechanisms of bacterial insusceptibility to biocides. Am J Infect Control. 2001;29:259-61.
- Thomas L, Maillard JY, Lamber RJW, Russell AD. Development of resistance to chlorhexidine diacetate in Pseudomonas aeruginosa and the effect of a ‘residual’ concentration. J Hosp Infect. 2000;46:297-303.
- Russell AD. Biocides and pharmacologically active drugs as residues and in the environment: is there a correlation with antibiotic resistance? Am J Infect Control. 2002;30:495-8.
- Gilbert P, Allison DG, McBain AJ. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J Appl Microbiol. 2002;92:98S-110S.
- Izano EA, Shah SM, Kaplan JB. Intercellular adhesion and biocide resistance in nontypeable Haemophilus influenzae Microb Pathog. 2009 Apr;46(4):207-13.
- Adams D, Quayam M, Worthington T, Lambert P, Elliott T. Evaluation of a 2% chlorhexidine gluconate in 70% isopropyl alcohol skin disinfectant. J Hosp Infect. 2005;61:287-90.
- Ghannoum M, O’Toole GA, editors. Microbial Biofilms. Herndon (VA): ASM Press. 2004:250-68.
- Smith K, Hunter IS. Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J Med Microbiol. 2008;57:966-73.
- Potera C. Forging a link between biofilms and disease. Science. 1999;283:1837-8.
- Toté K, Horemans T, Vanden Berghe D, Maes L, Cos P. Inhibitory effect of biocides on the viable masses and matrices of Staphylococcus aureus and Pseudomonas aeruginosa Appl Environ Microbiol. 2010 May;76(10):3135-42.
- Wong HS, Townsend KM, Fenwick SG, Trengove RD, O’Handley RM. Comparative susceptibility of planktonic and 3-day-old Salmonella typhimurium biofilms to disinfectants. J Appl Microbiol. 2010;108:2222-8.
- Vigeant P, Loo VG, Bertrand C, Dixon C, Hollis R, Pfaller MA, McLean APH, Briedis DJ, Perl TM, Robson HG. An outbreak of Serratia marcescens infections related to contaminated chlorhexidine. Infect Control Hosp Epidemiol. 1998;19:791-4.
- Marrie TJ, Costerton JW. Prolonged survival of Serratia marcescens in chlorhexidine. Appl Environ Microbiol. 1981 Dec;42(6):1093.
- Benson L, LeBlanc D, Bush L, White J. The effects of surfactant systems and moisturizing products on the residual activity of a chlorhexidine gluconate handwash using a pigskin substrate. Infect Control Hosp Epidemiol. 1990;11(2):67-70.
- Kawamura-Sato K, Wachino J, Kondo T, Ito H, Arakawa Y. Reduction of disinfectant bactericidal activities in clinically isolated Acinetobacter species in the presence of organic material. J Antimicrob Chemother. 2008;61:568-76.
- Frantz SW, Haines KA, Azar CG, Ward JI, Homan SM, Roberts RB. Chlorhexidine gluconate (CHG) activity against clinical isolates of vancomycin-resistant Enterococcus faecium (VREF) and the effects of moisturizing agents on CHG residue on the skin. J Hosp Infect. 1997;37:157-64.
- Karpanen TJ, Worthington T, Conway BR, Hilton AC, Elliott TSJ, Lambert PA. Penetration of chlorhexidine into skin. Antimicrob Agents Chemother. 2008 Oct;52(10):3633-6.
- Karpanen TJ, Worthington T, Conway BR, Hilton AC, Elliott TSJ, Lambert PA. Penetration of chlorhexidine into skin. Antimicrob Agents Chemother. 2009 Apr;53(4):1717-9.
- Prevention, Pesticides and Toxic Substances. R.E.D. Facts: Chlorhexidine Diacetate. EPA-738-F-96-025. Washington, DC: United States Environmental Protection Agency. 1996 Sep. 5 p.
- Lawrence JR, Zhu B, Swerhone GDW, Topp E, Roy J, Wassenar LI, Rema T, Korber DR. Community-level assessment of the effects of the broad-spectrum antimicrobial chlorhexidine on the outcome of river microbial biofilm development. Appl Environ Microbiol. 2008 Jun;74(11):3541-50.
- Dynes JJ, Lawrence JR, Korber DR, Swerhone GD, Leppard GG, Hitchcock AP. Quantitative mapping of chlorhexidine in natural river biofilms. Sci Total Environ. 2006 Oct 1;369(1-3):369-83.
- Chlorhexidine Gluconate MM. In: McEvoy GK, Snow EK, Miller J, Kester L, Welsh OH Jr, Heydorn JD, Le T, Mendham NA, O’Rourke A, Braun S, Grande KJ, Litvak K, editors. AHFS Drug Information [Internet]. Bethesda: American Society of Health-System Pharmacists, Inc. c2010; [cited 2010 Oct 11]; [about 4 screens]. Available from http://www.medscape.com/druginfo/monograph?cid=med&drugid=5356&drugname=Chlorhexidine+ Gluconate+MM&monotype=monograph. Subscription required to view.
- Mohammadi Z, Shahriari S. Residual antibacterial activity of chlorhexidine and MTAD in human root dentin in vitro. J Oral Sci. 2008;50(1):63-7.
- Wilson M. Susceptibility of oral bacterial biofilms to antimicrobial agents. J Med Microbiol. 1996;44:79-87.
- Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997 Aug 15;127(4):257-66.
- Pemberton LB, Ross V, Cuddy P, Kremer H, Fessler T, McGurk E. No difference in catheter sepsis between standard and antiseptic central venous catheters. A prospective randomized trial. Arch Surg. 1996 Sep;131(9):896-9.
- Mermel LA. Intravascular catheter-related bloodstream infection. Emerg Infect Dis. 2001;7(2):197-9.
- Oda T, Hamasaki J, Kanda N, Mikami K. Anaphylactic shock induced by an antiseptic-coated central venous catheter. Anesthesiology. 1997 Nov;87(5):1242-4.
- Terazawa E, Shimonaka H, Nagase K, Masue T, Dohi S. Severe anaphylactic reaction due to a chlorhexidine-impregnated central venous catheter. Anesthesiology. 1998 Nov;89(5):1298-300.
- Nhung DTT, Freydiere AM, Constant H, Falson F, Pirot F. Sustained antibacterial effect of a hand rub gel incorporating chlorhexidine-loaded nanocapsules (Nanochlorex). Int J Pharm. 2007 Apr 4;334(1-2):166-72.
- Lboutounne H, Chaulet JF, Ploton C, Falson F, Pirot F. Sustained ex vivo skin antiseptic activity of chlorhexidine in poly(ε-caprolactone) nanocapsule encapsulated form and as a digluconate. J Control Release. 2002 Aug 21;82(2-3):319-34.