The antimicrobial properties of alcohol were reported first for ethyl alcohol (EA) in 1888 and subsequently for isopropyl alcohol (IA) and N-propyl alcohol (NA) in 1904.1,2 Although alcoholic antimicrobial activity increases with increasing carbon chain length, these alcohols—also referred to as ethanol, isopropanol and N-propanol—are preferred for their solubility in water.2,3 The bactericidal activity of these compounds is typically ranked N-propanol > isopropanol > ethanol; however, only ethanol and isopropanol are approved in the United States for use in healthcare antisepsis and disinfection.2-6 In light of this, although N-propanol is widely used in Europe, this article focuses primarily on ethyl and isopropyl alcohol and attempts to provide an in-depth discussion of the attributes, efficacy, and role of these alcohols in preventing infection.
Mechanism of action
Alcohols are believed to exert their antimicrobial effects primarily by denaturing and coagulating proteins, leading to disruption of cellular metabolism and membrane lysis.2,6-10 The attraction between the carbon tails of alcohols and hydrophobic protein residues enables alcohols to insert themselves into proteins, where the polar presence of the alcoholic oxygen atom weakens lipophilic interactions between non-polar residues and increases the internal affinity of the structure for water. This decreases the stability of the 3-dimensional protein structure and causes enzymes to fall apart at physiologically relevant temperatures.11-16
Alcohols appear to permeabilize the cytoplasmic membrane through a similar mechanism.17 Although this mechanism of action has not been intensively studied, the microbicidal effects of cytoplasmic disruption and protein denaturation are validated by reports linking cell death with loss of protein viability.18,19 Ultimately, alcohol exposure destroys proteins and disorganizes the cell membrane, causing catastrophic damage that rapidly kills a variety of microbes.
Efficacy
Not surprisingly, the devastating protein denaturation induced by alcohol exposure renders ethanol and isopropanol highly effective antiseptic agents. In vitro, alcohols rapidly eliminate a wide variety of microorganisms and this activity has been shown to extend to in vivo tests as well, where alcohols are generally recognized as the most effective form of hand antisepsis.2 While they offer no protection against post-application contamination, alcohols have been shown to reduce bacterial counts on inanimate surfaces as well, providing solid rationale for their use as general healthcare disinfectants and antiseptics.
Unfortunately, while studies indicate that alcohols potently reduce microbial contamination, they have not been shown to have an independently superior effect on infection rates in either the community or healthcare setting. However, alcohols are easy to use, can be placed directly at point-of-care sites, and are equal to or better than other antiseptics at reducing microbial load, making them excellent options for effective, accessible antisepsis and disinfection.
In vitro spectrum
Ethyl and isopropyl alcohol are rapidly microbicidal against vegetative bacteria, fungi, and most viruses.2,6,7-9,20 This potency is highly concentration-dependent, with antimicrobial effectiveness beginning at concentrations of 30% and maximum efficacy typically achieved at concentrations of 60-95% ethanol and 70-91.3% isopropanol, though decreases in activity towards the higher end of these ranges have been observed.2,5-9,21 Activity is also enhanced as temperature increases from 10° C to 40° C.22 Against Gram-positive and Gram-negative bacteria, the 15-30 second in vitro reduction factors (RFs) of alcohols antiseptics typically range from 4-8 log10 colony-forming units (CFUs).23-30 Seventy-percent ethanol is highly effective against mycobacteria—including Mycobacterium tuberculosis—after 30 seconds of exposure and eliminates fungi within 15-20 minutes.23,31-34 High alcohol concentrations are active against enveloped and non-enveloped viruses, including human immunodeficiency virus (HIV), influenza A, SARS-coronavirus, and hepatitis B/C, though hepatitis A, poliovirus, and MS2 bacteriophage appear to be relatively insusceptible to alcoholic inactivation.2,23,30,35-47 Despite this broad antimicrobial spectrum, alcohols are not considered high-level disinfectants due to their lack of relevant sporicidal activity.7,48-54
In vivo results
In addition to their potent in vitro efficacy, alcohols also demonstrate significant antimicrobial activity in vivo. Since hands are the most significant target for reducing nosocomial infection, dozens of studies have explored the effects of alcohols on both transient and normal bacterial hand flora.6 In these studies, ethanol and isopropanol are associated with multilog RFs at a variety of concentrations and exposure times. Alcohols are also effective as disinfectants on a wide variety of surfaces, though specific RFs have not been extensively reported for inanimate surfaces.7 Unfortunately, this antiseptic and disinfecting efficacy is highly dependent on exposure time, and since reported in-use application times are typically far shorter than those used in most in vivo studies, log10 RFs seen in clinical practice may not reflect those observed in vivo.6 However, while the clinical relevance of most study times is debatable, alcohols are generally superior to other biocides in vivo and are widely considered the standard of reference for antimicrobial efficacy.
Antisepsis
The antiseptic effects of ethyl and isopropyl alcohol are well-established in the literature. After a comprehensive literature review, Kampf et al (2004) estimated that ethanol and isopropanol reduce transient bacterial flora by 2.6-4.5 and 4-6.81 log10, respectively, and resident flora by 1.5-2.4 log10.2 An independent analysis of more than 30 studies and over 2,000 data points generally corroborates these ranges (Tables 1 and 2), which appear to be accurate regardless of the method used to contaminate hands.55-57 Alcohols are also effective against viruses in vivo, and are associated with RFs of 0.9-4.64 log10 at 10-30 second exposure times.58-62 Although the results of in vivo trials are not entirely consistent, the bacterial and viral RFs of alcohols are generally superior to the RFs of other antiseptics, leading the CDC to recommend alcohols as the preferred agents for general hand antisepsis.6,63
Like most biocides, the efficacy of alcohols is highly dependent on exposure time. Unfortunately, clinical handwashing duration is typically less than 10 seconds, and most in vivo studies evaluate the efficacy of alcohols at antisepsis times ranging from 30-60 seconds.6 To address this discrepancy, Sickbert-Bennett et al evaluated the effects of 61% ethanol on transient Serratia marcescens after 10 seconds of exposure.64 Unlike the 2-4 log10 RFs seen at 30-60 seconds of antisepsis, 10 seconds of 61% ethanol use was associated with a 1.55 log10 RF in bacterial load and actually performed worse than 4% chlorhexidine (1.89 log10 RF), 1% triclosan (1.9 log10 RF), plain soap (1.87 log10 RF) and tap water (2 log10 RF). Similarly, another trial reported a 2.15 log10 RF for 70% isopropanol on Escherichia coli at 10 seconds of exposure, although isopropyl alcohol outperformed chlorhexidine (1.96 log10 RF), plain soap (0.5 log10 RF) and tap water (1 log10 RF).61
These findings indicate that alcoholic antisepsis may not provide the same superior antisepsis observed in most in vivo tests under in-use application times. In contrast, the results of a study by Dharan et al suggest that 15-30 seconds of exposure to alcohols may be associated with even greater effects on transient bacteria (5.03-7.18 log10 RF) than previously described.65 However, due to differences in the contamination, disinfection and recovery techniques employed by Dharan and colleagues, these results were excluded from the mean RF value calculations in the following table. Inclusion of this study increases the mean RFs of ethanol and isopropanol to 5.74 (range 2.7-6.8) and 5.66 (range 5.03-6.05) log10 RF, respectively, at 15 seconds application time and 5.37 (range 1.97-6.88) and 4.43 (range 2.9-6.81) log10 RF, respectively, after 30 seconds. Although this study paints alcoholic antisepsis in an even more favorable light, the aberrant nature of the reported RFs also highlights the risk of under or overestimation of in vivo efficacy inherent in current testing methodology.
Further exposing the intrinsic variability of in vivo tests, several studies report unusually low RFs associated with alcoholic antisepsis. In one trial, 61% ethanol was completely ineffective (-0.2 to 0 log10 RF) in eliminating the spores of a Bacillus anthracis surrogate, though this result is not surprising since alcohols lack sporicidal activity in vitro.66 The results of two other studies comparing ethanol with a surfactant, allantoin and benzalkonium chloride (SAB) antiseptic are more difficult to explain. After a 2-minute application time on hands artificially contaminated with Serratia marcescens, both SAB antiseptic and 62% ethanol were associated with a 2.6-2.8 log10 RF, while 62% ethanol gel was associated with a 2.1 log10 RF.67,68 Since ethanol and ethanol gel are associated with a 3-4 log10 reduction of transient bacteria after 60 seconds of exposure, these results seem largely incongruous with established literature.4,69 The authors fail to address this inconsistency in their article, calling the quality of their study into serious question. Aside from these few studies, however, alcohols perform quite well in most trials and the weight of evidence seems to favor their use as antiseptics at 30 second exposure times.70
In addition to their proven antiseptic efficacy, alcohols have significant disinfecting activity. Alcohols are highly flammable and are not recommended for disinfection of large environmental surfaces; however, they can be safely used to disinfect a variety of inanimate objects.62,103,104 The efficacy of alcoholic disinfection been examined on a variety of medical devices as well as a slew of more routine objects, where ethanol and isopropanol generally demonstrate excellent antimicrobial activity against bacteria and fungi.62,105 Unfortunately, alcohols have poor activity against sporulated bacteria and some viruses, leading to the recommendation that ethanol and isopropanol only be used to disinfect noncritical healthcare objects.104
Medical devices are one of several target healthcare vectors for alcohol-based disinfection. In several trials, 70% alcohol outperformed iodine and quaternary ammonium compounds (QACs) and was equivalent or superior to phenolics and formaldehyde for disinfecting oral thermometers, reducing contamination rates by 79.6-97%.106-108 In contrast, coliform bacteria persisted on 71.2% of contaminated rectal thermometers wiped down with 95% ethanol and soap.109 Alcohols have also been tested for use in the disinfection of endoscopes, which have been linked to more infection outbreaks than any other medical instrument.104,110-115 Following 5 minutes of immersion in benzalkonium chloride, immersion of endoscopes in 96% alcohol for 5 minutes is associated with complete elimination of vegetative bacteria.116 However, due to the poor activity of alcohols against spores, alcoholic disinfection of endoscopes is limited to rinsing after high-level disinfection.114,117,118
Further studies have evaluated the efficacy of alcohols for disinfection of intravascular transducers and ophthalmologic tonometers, both of which have been implicated in nosocomial infection outbreaks.119,120 For transducers, wiping with 70% alcohol sterilizes 98% of the blood pressure monitors heads and is equivalent to ethylene oxide in terms of positive culture risk and patient outcomes.121,122 Alcohols also eliminate over 3 log10 of type 1 human immunodeficiency virus (HIV), coronavirus 229E, adenovirus type 5, and parainfluenzavirus type 3 from tonometers and other non-porous surfaces.123,124 However, 70% isopropanol is ineffective at eliminating adenovirus 8 (0.47-1.07 log10 RF)—a common cause of epidemic keratoconjunctivitis—from inanimate objects and is not recommended for disinfection of tonometers or other ophthalmologic devices.120,125,126
Alcohols have also been evaluated as disinfectants for a variety of noncritical healthcare objects. In a study of hospital staff pagers, swabbing with 70% ethanol was associated with a 1.22 log10 RF in bacterial contamination.127 Similarly, wiping ultrasound probe heads with 70% ethanol-moistened paper reduced bacterial load by 2.31 log10 CFUs.128 Other studies have reported that 89.3% of contaminated scissors and 100% of contaminated stethoscopes swabbed with 70% ethanol are sterile after disinfection.129,130 Wipe down with 70% alcohol has also been shown to reduce overall herpes simplex virus recovery from CPR manikin heads by 84%, completely eliminating the virus from all but the least accessible test sites.131 Although limitations in scope of effect prevent alcohols from being considered high-level disinfectants, these studies indicate an important role for alcohols in the disinfection of a wide variety of potential fomites.
Persistent activity
One often-mentioned weakness of alcoholic antisepsis is a lack of persistent activity. Defined by the CDC as “prolonged or extended antimicrobial activity that prevents or inhibits the proliferation or survival of microorganisms after application of the product,” persistent activity is facilitated by the presence of residual biocide on a surface after the initial application.6 This persistence is characterized by either continued suppression of surviving bacteria or activity against post-application inoculation. Alcohols are highly volatile and quickly evaporate under normal conditions, making them ideal for rapid antisepsis or disinfection; however, they leave no residue and provide no protective long-term antimicrobial effect. Despite this lack of residual presence, alcohols mildly suppress the regrowth of resident hand flora, but they offer no defense against exogenous recontamination. Unfortunately, since clean skin is quickly reinoculated by contact with contaminated surfaces, the antiseptic effects of alcohols are short-lived under most conditions.
Suppression of normal flora
While antisepsis substantially reduces the number of viable bacteria on the hand, it does not leave skin sterile. Even without external contamination, normal flora regrowth leads to recovery of bacterial load within hours of antisepsis. Bacterial regrowth is particularly important under surgical conditions, where contamination from skin surrounding the surgical site or glove perforation can lead to contamination of the surgical site and subsequent infection. To prevent this, the FDA has mandated that surgical scrubs must maintain hand flora below baseline for at least 6 hours after application.5 Alcohols meet this criterion through two mechanisms: 1) they have a mild post-antiseptic effect in vivo, and 2) they produce a decrease in bacterial load that cannot be overcome within 6 hours. Additionally, chlorhexidine gluconate (CHG) is sometimes added to ethyl or isopropyl alcohol in an effort to increase the longevity of their effects on hand flora, though the superiority of this combination is debatable. However, even in the absence of chlorhexidine, alcohols appear to independently inhibit bacterial regrowth long after their initial application.
Resident hand flora recovers by 0.49 log10 CFUs (weighted mean, range -0.29 to 1.3) within 3 hours of a 180-second application of an alcoholic surgical scrub.84,88,93 Further, a trial of the long-term effects of 61% ethanol reported that despite an initial 2.22 log10 RF, bacterial counts returned to baseline levels within 8 hours.101 In contrast, a trial by Mulberry et al found that 61% ethanol use was associated with a 1.1 log10 immediate RF in general hand flora and a 1.4 log10 RF three hours after antisepsis. Six hours after exposure, however, bacterial counts were only 0.5 log10 lower than baseline.100 Similarly, another study reported an increased RF (2.77 vs 2.48 log10) on gloved hands 3 hours after application of an 85% ethanol gel.88 This post-antiseptic effect may be due to sublethal damage to surviving bacteria that can be overcome on a culture medium, but results in further bacterial death on skin, a hypothesis that may also explain the slow initial regrowth rates observed in these studies.132
Chlorhexidine gluconate, a biguanide antiseptic with very low volatility, is frequently added to alcohol-based products in an effort to improve both immediate and long-term RFs. Problematically, while 93% of applied chlorhexidine remains on the skin for 5 hours, studies of alcoholic chlorhexidine at a 3 minute application time indicate that resident hand flora recovers by approximately 0.83 log10 CFUs within 3 hours of exposure and by 1.6 log10 after 6 hours.84,94,133 In 1 study, Mulberry et al reported initial, 3-hour and 6-hour RFs of 2.5 log10, 2.9 log10, and 2.5 log10, respectively, against general hand flora; however, it should be noted that many chlorhexidine studies suffer from inadequate validation of utilized neutralizing agents, potentially causing a 2 log10 overestimation of antiseptic efficacy.4,100,134-137 Regardless of neutralization validation, alcoholic chlorhexidine appears to lack any advantage over plain alcohol in terms of immediate effect and is often associated with a more rapid recovery of bacterial load, seriously undermining arguments favoring the use of this antiseptic combination.
Alcohols, alone or in combination with chlorhexidine, have also been shown to suppress general flora on other clinically relevant skin sites. Since baseline microbial loads for these sites are several log10 lower than typical hand counts, alcoholic antisepsis results in near-complete bacterial elimination, making recovery more difficult and thus amplifying the long-term effects of alcohols. In 4 trials, inguinal or abdominal antisepsis with 70% isopropanol led to a mean 2.78 log10 initial RF, 2.58 log10 6-hour RF and a 2.09 log10 24-hour RF. Addition of 2% chlorhexidine increased these values to 2.87 log10 initial RF, 2.76 log10 6-hour RF and 2.89 log10 24-hour RF.138-140 By comparison, using 89.5% N-propanol on the arms or back caused a mean 2.1 log10 initial RF and 0.35 log10 72-hour RF, whereas general skin flora remained 1.27 log10 lower than baseline at 72 hours when N-propanol was supplemented with 2% chlorhexidine.141 In contrast, while 60% isopropanol initially reduced inguinal carriage of Proteus mirabilis by 2.15 log10, 4% chlorhexidine had no meaningful effect and bacterial load for both agents returned to baseline within 4 hours.142 The combined results of these trials indicate that alcohols have a temporary suppressive effect on normal flora that may be mildly improved in combination with chlorhexidine.
Contamination prevention
Unfortunately, while alcohols appear to exert suppressive effects on surviving flora, the long-term effects of alcohols are also countermanded by exogenous contamination. Studies of fingertip recontamination after hand antisepsis report that healthcare staff acquire an average of 16-20 CFUs/minute during routine patient care.143,144 Assuming an average fingertip surface area of 1 cm2, the recontamination rate for the palmar surface of a whole hand may exceed 150 CFUs/minute, an estimate that is supported by reports that nurses may acquire from 100-6000+ CFUs during brief, ostensibly “clean” activities such as lifting, touching or taking a pulse from an infected patient.145,146 Baseline hand counts range from 3.9 x 104 to 4.6 x 106 total bacteria and should be reduced to 10-400 bacteria by alcoholic antisepsis.2,147 Using these values, regrowth times for general flora and a linear contamination rate of 150 CFUs/minute, hand flora will recover by 0.74-2.19 log10 CFUs within 10 minutes and reach 3.9 x 104 bacteria approximately 90 minutes after antisepsis.94
This rapid recontamination rate highlights the primary weakness of alcohol-based antisepsis: a lack of protective effect. Lowbury et al reported greater post-application inhibition of an inoculum of 5.1 x 103 Staphylococcus aureus after plain soap handwashing (0.12 log10 RF) than after use of 70% ethanol (0.03 log10 RF). Inoculation with 78,500 bacteria resulted in a 0.25 log10 RF for plain soap and a -0.01 log10 RF for ethanol.82 Similarly, Herruzo et al described a mean post-application RF of 0.3 log10 for 60% isopropyl alcohol against inoculums of Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. A combined alcohol-quaternary ammonium compound-chlorhexidine product, however, had a mean 3.9 log10 residual RF.78 Alcoholic chlorhexidine was also associated with a 4 log10 post-application RF in another study that showed no residual activity for 60% isopropanol, although both trials failed to sufficiently validate their neutralizing agents.148 These studies demonstrate the limitations of alcoholic antisepsis and indicate the potential benefits to supplementation with a less volatile antimicrobial.
Clinical trials
Despite their lack of protective effect, in vivo studies generally indicate that alcohols are superior to most other antiseptics. In an effort to link this efficacy to clinical outcomes, alcohols have been studied in a variety of trials evaluating their effects on hand contamination and infection rates in surgical, general healthcare and community settings.6 Although many of these studies suffer from limitations in design, size and scope, they generally fail to directly link alcoholic antisepsis to the ultimate goal of hand hygiene: reduced infection.149 Instead, the evidence indicates that under general use conditions, alcohols may reduce infection rates chiefly by improving handwashing compliance. Unlike soaps, alcohols are effective without water and can be strategically placed to facilitate point-of-care antisepsis. As a result, while the superiority of alcoholic antisepsis is unproven, alcohols increase ease of use and, by extension, handwashing compliance. This explanation helps to explain the conflicting results of efficacy trials and offers rationale for the continued use of alcohols despite disappointing clinical outcomes.
Surgical scrubbing
While numerous trials verify the in vivo effects of isopropanol and ethanol on perioperative bacterial counts, there is a lack of evidence to prove that alcoholic antisepsis reduces infection rates to a greater extent than other surgical scrubs. Since conducting a placebo-controlled trial on the effects of alcohols on nosocomial infection rate is ethically unreasonable, most studies compare antiseptic agents for effects on either microbial load or infection rate.150 However, in a test of antiseptic activity on murine surgical sites inoculated with highly virulent pneumococcal bacteria, ethanol and isopropanol decreased mortality from 100% to less than 15%.151 Unfortunately, clinical results are far less conclusive in humans. Although alcoholic antisepsis appears to provide superior perioperative reductions in hand contamination, the long-term effects of alcohols on either baseline bacterial load or surgical site infection rates appear to be equivalent to other antiseptic agents.
As preoperative antiseptics, the clinical effects of alcoholic antisepsis on bacterial load appear to be generally superior to most antiseptic agents. In one study, rubbing with 45% isopropanol/30% N-propanol/0.2% mecetronium etisulfate for 3 minutes reduced hand colonization by 2.05 log10 CFUs immediately and maintained a 1.66 log10 RF through the end of a 15-60 minute surgery, as compared to a 1.58 log10 immediate and 1.12 log10 post-operative RF using 7.5% povidone-iodine (P = 0.02-0.04).152 A separate study reported a 2.4 log10 initial and 1.8 log10 post-surgery RF for the same alcohol-preservative combination, whereas 4% chlorhexidine was associated with a mere 1.3 log10 initial and 0.9 log10 post-operative RF (P < 0.001).77 In another study, alcohols demonstrated better post-operative dampening of bacterial regrowth than 7.5% povidone-iodine or 4% chlorhexidine (4.7 vs 5.2 log10 CFUs, P = 0.017) at surgery times > 3 hours.153 These results show that despite a lack of persistent presence on the skin, alcohols are clinically equivalent or superior to other surgical scrubs in terms of perioperative bacterial counts.
In spite of their superior effects on hand counts, alcohols do not offer a clear benefit over other antiseptic agents in terms of baseline bacterial load or the incidence of surgical-site infections. Three weeks of antisepsis using 1% chlorhexidine in 61% ethanol has been shown to reduce prescrub flora by 0.54 log10 CFUs; however, use of 4% chlorhexidine also reduced baseline counts by 0.51 log10 CFUs (P = 0.08).154 In another hospital study, changing to a 3-minute, 70% ethanol-based rub slightly increased the incidence of surgical site infection (1.33% vs < 1%).155 The clearest data stems from a 16-month clinical trial comparing the 30-day incidence of surgical site infection (SSI) after antisepsis with 45% isopropanol/30% N-propanol/0.2% mecetronium etisulfate or either 4% povidone-iodine or 4% chlorhexidine. In this crossover study, infection occurred after 53 of 2,135 surgeries (2.44%) when hands were sanitized using the alcohol-preservative combination and after 55 of 2,252 surgeries (2.48%) using standard surgical scrubs.156 The incidence of SSIs was statistically equivalent for alcohols, povidone-iodine and chlorhexidine (P < 0.01). Based on these results, there appears to be poor correlation between reduction of hand flora and decreases in infections. Alcohols are more effective on bacterial load than other antiseptics; however, that efficacy does not directly translate into fewer surgical infections.
Hygienic hand antisepsis
Alcohols have also been widely studied as general antiseptics in both the healthcare and community setting. As general healthcare antiseptics, the effects of alcohols on bacterial load and nosocomial infection rates are similar to those reported for their use as surgical antiseptics. Hand antisepsis with alcohol-based products is associated with lower baseline bacterial load than reported for plain soap; however, alcohols have not been shown to independently reduce hospital-acquired infections.143 Several clinical trials have also shown benefit to implementation of an alcohol-based hand hygiene program, but these results appear to be largely due to increased handwashing compliance rather than improved antiseptic efficacy. Similarly, alcohols also appear to reduce community-acquired infections by facilitating quick, accessible hand sanitization, although the quality of most community-based studies is debatable and comparison studies between alcohols and other antiseptics are lacking.157 Overall, while alcohols reduce hand contamination to a greater extent than plain soap, their impact on healthcare and community infection rates is mild and appears to derive mainly from their beneficial effects on handwashing compliance.
Contamination
In terms of bacterial elimination, alcoholic antisepsis has repeatedly demonstrated superiority over plain soap, although proof that alcohols are more efficacious than other antiseptics is limited. Alcohol is more effective than soap at ridding hands of Gram-negative bacteria, and has been shown to prevent transfer of Gram-negative bacteria from contaminated hands to sterile catheters in 10/12 experiments, while plain soap was effective in only 1/12 tests.146,158 Prior to training in antiseptic technique, glycerol in 70% isopropanol gel and plain soap were associated with respective 0.52 log10 and 0.05 log10 RFs, which increased to 0.68 log10 and 0.4 log10 RFs, respectively, with technique standardization.159 In a Russian neonatal intensive care unit (ICU), use of a 79% ethanol/0.1% quaternary ammonium compound hand rub instead of plain soap reduced the Klebsiella pneumoniae colonization rate from 21.5 colonized patients/1,000 patient days to 3.2 patients/1,000 patient days. The colonization rate for other pathogens—especially Candida albicans—was also reduced, albeit to a lesser extent.160 In other studies, alcoholic antisepsis reduced pathogen isolation from 68% to 25% on hands with artificial fingernails and substantially reduced transient organism recovery on the hands of nurses wearing rings.161,162 These results validate the current preference for alcoholic antisepsis over handwashing with plain soap.6
In addition to the preceding studies, 45% isopropanol/30% N-propanol/0.2% mecetronium etisulfate has proven superior to plain soap antisepsis in multiple European hand contamination studies. In 4 trials, this combined alcohol-preservative product was associated with a 0.93-1.7 log10 RF on fingertips, which was significantly greater than the 0.3-0.74 log10 RF decrease in contamination observed with soap (P ≤ 0.0003).163-166 Another trial actually reported a 0.12 log10 increase in bacterial load for plain soap versus a 0.34 log10 decrease for the alcohol-antiseptic, while a study of infection control professionals using 45% isopropanol/30% N-propanol/0.2% mecetronium etisulfate reported a mean RF of 2 log10 (range 0-3.85 log10).167,168 For both soap and the alcohol-based product, however, bacterial counts appear to return to baseline within 10-30 minutes of antisepsis.163 Although N-propanol is not approved for use in the United States, these well-designed trials are included here as further evidence of the superiority of alcohol-based antisepsis on bacterial load.
Despite their generally accepted superiority over plain soap, alcohols do not offer a clear clinical advantage over other antiseptics. For example, a 4-week study reported no effect on baseline nurse hand flora for either 60% isopropanol or 4% chlorhexidine.169 Alcohols are also non-superior to other antiseptics for reducing false-positive rates during blood culture sampling and are statistically equivalent to other antiseptics in interrupting the transfer of bacteria from contaminated hands to sterile fabric.170,171 Furthermore, 1 study reported a 1.4 log10 fingertip RF following use of 45% isopropanol/30% N-propanol/0.2% mecetronium etisulfate, whereas handwashing with 10% povidone-iodine or 4% chlorhexidine resulted in a 1.13-1.2 log10 RF. This difference was statistically irrelevant.164 In contrast, a trial of hand antisepsis during patient care reported a significantly greater (P = 0.012) median reduction in bacterial load after a 30-second application of this alcohol-antiseptic than after use of 4% chlorhexidine (0.77 log10 vs 0.49 log10 RF).172 Although alcohols do not appear to offer a definitive advantage over other antiseptics, it should be noted that there is a conspicuous lack of quality trials comparing the effects of various agents on hand contamination. Based on the available data, however, alcoholic antisepsis appears to be superior to plain soap handwashing, but its place in the hierarchy of antiseptic efficacy has yet to be established.
Healthcare infection rates
Similar to their effects on hand contamination, there is a lack of clear evidence that alcohols independently reduce infection rates to a greater extent than other forms of hand antisepsis. While many studies show an infection-rate benefit to alcohol-based antisepsis, the results of these studies are complicated by a simultaneous increase in handwashing compliance. Since a 12-40% improvement in handwashing compliance—independent of changes in antiseptic regimen—has been shown to decrease nosocomial infections by 41-45%, the direct effects of alcoholic antisepsis on infection rates are difficult to extricate from an increase in compliance.173,174 A 5-year trial reported a 57% reduction in cases of methicillin-resistant Staphylococcus aureus (MRSA) bacteremia 3 years after initiating a hand hygiene program utilizing alcoholic chlorhexidine (P = 0.01).175 However, hand hygiene compliance doubled (21% vs 42%) during the first 12 months of the study. Improved compliance (56% to 95%) also confounded the results of another trial, where conversion from 2% chlorhexidine to an alcohol foam reduced the rate of Clostridium difficile colitis from 4.96 infections/10,000 patient days to 3.98 infections/10,000 patient days (P = 0.0036).176 While these studies demonstrate that alcoholic antisepsis can positively affect infection rate, this benefit may be due to improved handwashing compliance rather than superior effects on bacterial load.
The hypothesis that increased compliance is the primary factor driving decreased infection rate is further supported by the results of trials with stable handwashing compliance. In a 2-year crossover trial of 2,826 infants in 2 neonatal ICUs, alcoholic antisepsis was associated with 12.1 infections/1,000 patient days. Handwashing with 2% chlorhexidine was associated with 9.5 infections/1,000 patient days. However, after adjusting for birth weight, study site, surgery and follow-up time, both biocides were statistically equivalent.177 Similarly, an 8-month crossover trial of 1,894 patients in 3 ICUs reported 38 infections/1,000 patient days when 4% chlorhexidine was used and 50.7 infections/1,000 patient days with the use of 60% isopropanol. This difference, however, was statistically nonsignificant and did not affect length of stay or mortality.178 During a 1-year interventional study with stable handwashing compliance, MRSA infections decreased by less than 5% and vancomycin-resistant Enterococcus (VRE) infections actually increased by 12-13% despite a doubling in use of an alcohol foam product.179 On the other hand, 2 trials reported no difference in hospital-acquired infection rate between alcoholic antisepsis and plain soap handwashing despite near-doubled compliance.158,180 These results indicate that compliance may be a better, albeit imperfect, independent predictor of nosocomial infections than use of alcohol-based products.
Internationally, the increased handwashing facilitated by alcohol-based antiseptics has been shown to dramatically reduce nosocomial infections under conditions where soap-and-water handwashing is impractical. Nosocomial infections fell from 13.1% to 2.1% in a Vietnamese trial after implementation of an alcohol-centered handwashing program as handwashing compliance increased from approximately 0% to 43-71%.181 In another Vietnamese trial, SSIs decreased from 8.3% to 3.8% with use of a 70% isopropanol/0.5% chlorhexidine antiseptic, whereas SSIs increased from 7.2% to 9.2% in the control group. Although compliance rates were not reported, baseline hand antisepsis rates were likely low, since only 1 sink was available in the 60-bed surgical ward.182 By comparison, during an 8-year Norwegian trial of 27,284 patients, the prevalence of nosocomial infections only fell from 7.9% to 7.1% while use of an ethanol antiseptic increased 7-fold (P = 0.04).183 Taking these findings into account, it seems likely that alcohols reduce nosocomial infections primarily by increasing the speed and accessibility of antisepsis, an effect that becomes more apparent when handwashing compliance is low.
Community infection rates
Although less research has been conducted regarding the effects of alcoholic antisepsis on infection rates in the community than in healthcare, available evidence indicates that regular use of alcohols may decrease illness. As in the healthcare environment, this benefit appears to stem largely from increased handwashing compliance. The effects of alcoholic antisepsis on community-acquired infections have been studied most in elementary schools, where transmission of infectious pathogens increases student and parental absenteeism from school and work.184 Despite repeated instruction and encouragement, handwashing compliance is remarkably poor among school-age children. For instance, only 54% of middle- and high-school students wash their hands after using the restroom, 37% wash for > 5 seconds and a mere 18% use soap.185 By facilitating improved handwashing compliance among students, alcoholic antisepsis has the potential to substantially reduce scholastic infection rates. For example, in a study of 290 kindergarten-to-3rd grade students in 5 schools, classrooms equipped with a bottle of 62% ethanol reported 1.05 illness-related absences/100 student days, whereas classes without hand sanitizer had only 2.08 absences/100 student days.186 Similarly, a controlled study of 138 students reported a 28% reduction in absences due to illness after installation of an alcohol dispenser.187
Despite the positive finding of these trials, increased access to alcohol-based hand sanitizers is not conclusively associated with decreased illness.157 The strongest evidence comes from a trial incorporating 6,080 students from 4 states, 5 school districts, and 18 schools. In this trial, classroom use of 62% ethanol reduced year-long student absences by 19.8% and teacher absenteeism by 10.1%. While these overall results were statistically significant, analysis of individual school districts showed that only 3 of 5 districts actually reached a statistically relevant reduction in student absenteeism. One district actually reported a nonsignificant 3.75% increase in absences with use of the alcohol product.188 Similarly underwhelming results have also been reported in several other trials. In a study of 253 students, classes with alcohol dispensers averaged 3.45 absences/100 student days, while control classes averaged 3.75 absences/100 student days.189 A study of 360 second-to-third grade students reported no difference in absenteeism between plain soap and alcoholic antisepsis.190 Further, while an 8-week study of 285 students reported a statistically significant 8% decrease in school absences due to gastrointestinal illness with use of 70% ethanol, it also reported a nonsignificant 2% increase in respiratory illness for the test group.191 Based on these results, alcoholic antisepsis does not appear to be the cure for the common cold; however, increases in hand hygiene facilitated by alcohol use may help reduce community infection rates.192
Hindering factors
A variety of factors may help to explain the lack of correlation between the in vitro, in vivo and clinical efficacy of alcohol-based antisepsis. As mentioned previously, in-use application times are typically too short to eliminate hand flora to a greater extent than plain soap handwashing.6,64 Applied volume and inoculum size are also important considerations, since 3 mL of alcohol has been shown to cause a 0.27-1.28 log10 greater RF than 1 mL of alcohol and increasing bacterial load from 103 to 106 CFUs/fingertip reduces alcoholic RFs by 0.24-1.83 log10.72,73,91 Furthermore, practical application technique rarely resembles the regimented procedures used during in vivo tests. One recent study reported that only 31% of tested healthcare workers properly applied an alcohol hand rub, resulting in a mere 1.4 log10 RF. After hand rub training, antibacterial efficacy rose to 2.2 log10 RF.193 Other personnel-related factors include artificial fingernails or ring wearing, which have been shown to significantly decrease the effectiveness of alcoholic antisepsis.161,162 Overall, these factors seriously undermine the efficacy of alcohols and may limit their clinical effectiveness.
In addition to personnel-related factors, alcohols have several inherent limitations. Alcohols affect microbes by disrupting proteins and are inhibited by extraneous protein, leading to recommendations against their use on visibly dirty surfaces.6-9,20,32,33,103 Product formulation may also reduce efficacy, since alcohol gels appear to be about 1-2 log10 less effective in vivo than their rinse counterparts (see Table 1).64,76,77,87,166,194 Furthermore, dried bacteria are less susceptible to alcohols, potentially enabling desiccated microbes to better survive surface disinfection.21,49 Infection outbreaks have been linked to alcohol solutions contaminated with Bulkholderia cepacia—which has an unusual ability to metabolize alcohols—and sporulated Bacillus cereus.195,196 Lastly, while not related to clinical efficacy, the flammability of alcohols also limits their use as disinfectants.103 At concentrations ≥ 70%, ethyl and isopropyl alcohol have flash points lower than 21° C and are considered “easily flammable;” however, the reported incidence of alcohol-related fires is extremely low.6,70,197 Failure to account for these limitations may also help to explain the disconnection between in vivo and clinical results.
Biofilms
Another hindering factor of alcoholic antisepsis and disinfection is poor efficacy against biofilms. Biofilms form readily on almost any surface and are associated with an estimated 65% of nosocomial infections, making them a significant consideration in infection prevention.198-200 Unfortunately, an in vitro study reported that 1 minute of exposure to 70% alcohol reduced the number of viable biofilm Pseudomonas aeruginosa and Staphylococcus aureus by a mere 0.59-0.96 log10 and 0.96-1.22 log10 CFUs, respectively. After a full hour, ethanol decreased both strains by 1.4-2 log10 CFUs and isopropyl alcohol reduced Staphylococcus aureus by only 1.9 log10 CFUs. However, 70% isopropanol was relatively effective against Pseudomonas aeruginosa, causing a > 5 log10 RF after 30 minutes of exposure.201 Isopropanol also appears to be efficacious against Staphylococcus epidermidis biofilms, killing 5.3 log10 bacteria within 30 seconds.202 In contrast, 70% ethanol only reduces sessile Salmonella Typhyimurium by about 2 log10 CFUs after 1 and 5 minutes of exposure.203 Furthermore, fungal biofilms also demonstrate decreased susceptibility to ethanol.204 Given the ubiquitous nature of biofilms, the generally poor efficacy of alcohols against sessile microorganisms may seriously undermine the clinical impact of these disinfectants.
Toxicity
While the clinical efficacy of alcohols is somewhat limited by personnel-related factors, the presence of organic material and biofilms, toxicity does not appear to be a significant hindrance to their use. Unfortunately, despite their excellent safety profile, alcohols suffer from the popular belief that they damage skin.205 This stigma is likely derived from the burning sensation experienced when alcohols are applied to pre-damaged skin.206 Alcohols do solubilize sebum and lipids in the epidermis; however, the majority of studies indicate that alcohol-based antisepsis is less drying and better tolerated than plain soap or other antiseptics.23,67,154,167,169,177,207 Additionally, alcohols can be combined with a variety of emollients to reduce skin irritation and most healthcare workers consider them to be acceptable for routine use.155,163,166,208,209 Since the Environmental Protection Agency (EPA) considers ethanol to be practically non-toxic to both humans and animals, alcohols appear to be environmentally and clinically safe antimicrobial agents.210
Conclusion
After more than a century of scrutiny and research, alcohols remain among the safest, most effective biocides available and are used for a remarkably wide variety of disinfecting and antiseptic purposes. Given their potent, largely irresistible mechanism of action, broad antimicrobial spectrum and superior in vivo efficacy, alcohols appear to deserve their status as the preferred antiseptic agent. Unfortunately, in spite their many strengths, ethanol and isopropanol have not been shown to independently reduce infection rates. Rather, they appear to mitigate infection primarily by improving handwashing compliance. Since the greatest weakness of alcohol-based antisepsis and disinfection appears to be a lack of residual presence, efforts to further reduce community and nosocomial infection rates should focus on providing inter-application antimicrobial activity. Until a product with protective, persistent activity reaches the market, however, alcohols will likely remain the most effective and accessible antimicrobial agent available.
References
- Wirgin G. Vergleichende Untersuchungen überdie keimabtötenden und die entwicklungshemmenden Wirkungen von Alkoholen der Methyl-, Äthyl-, Propyl-, Butyl-, und Amylreihen. Z Hyg. 1904;46:49-168.
- Kampf G, Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin Microbiol Rev. 2004 Oct;17(4):863-93.
- Tilley FW, Schaffer JM. Relation between the chemical constitution and germicidal activity of the monohydric alcohols and phenols. J Bacteriol. 1926;12(5):303-9
- Rotter ML. Hygienic hand disinfection. Infect Control. 1984;5:18-22.
- Food and Drug Administration. Tentative final monograph for healthcare antiseptic products: proposed rule. Federal Register. 1994;59:31441-52.
- Boyce JM, Pittet D; Healthcare Infection Control Practices Advisory Committee; HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hygiene Task Force. Am J Infect Control. 2002 Dec;30(8):S1-46.
- Rotter ML. Hand washing and hand disinfection. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control, 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins. 2004:1727-46.
- Russell AD, Chopra I. Understanding Antibacterial Action and Resistance, 2nd ed. Hertfordshire, England: Ellis Horwood. 1996. 292 p.
- McDonnell G, Russell DA. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999 Jan;12(1):147-79.
- Cheeseman KE, Denyer SP, Hosein IK, Williams GJ, Maillard JY. Understanding the lethal effect of alcohol hand rubs: an in vitro study with Staphylococcus aureus J Hosp Infect. 2010 Nov;76(3):264-6.
- Sturtevant JM, Velicelibi G. Calorimetric study of the interaction of lysozyme with aqueous 1-propanol. Biochemistry. 1981 May 26;20(11):3091-6.
- Velicelibi G, Sturtevant JM. Thermodynamics of the denaturation of lysozyme in alcohol-water mixtures. Biochemistry. 1979 Apr 3;18(7):1180-6.
- Brandts JF, Hunt L. The thermodynamics of protein denaturation. 3. The denaturation of ribonuclease in water and in aqueous urea and aqueous ethanol mixtures. J Am Chem Soc. 1967 Sep 13;89(19):4826-38.
- Schrier EE, Ingwall RT, Scheraga HA. The effect of aqueous alcohol solutions on the thermal transition of ribonuclease. J Phys Chem. 1965 Jan;69(1):298-303.
- Hermans J Jr. The effect of protein denaturants on the stability of the α-helix. J Am Chem Soc. 1966 Jun 5;88(11):2418-22.
- Parodi RM, Bianchi E, Ciferri A. Thermodynamics of unfolding of lysozyme in aqueous alcohol solutions. J Biol Chem. 1973 Jun 10;248(11):4047-51.
- Maillard JY. Bacterial target sites for bactericidal action. J Appl Microbiol. 2002;92:16-27S.
- Sykes G. The influence of germicides on the dehydrogenases of coli. I. The succinic acid dehydrogenase of Bact. coli. J Hyg (Lond). 1939 Jul;39(4):463-9.
- Dagely S, Dawes EA, Morrison GA. Inhibition of growth of Aerobacter aerogenes: the mode of action of phenols, alcohols, acetone and ethyl acetate. J Bacteriol. 1950 Oct;60(4):369-78.
- Damani NN. Manual of Infection Control Procedures, 2nd ed. London: Greenwich Medical Media Limited. 2003. 333 p.
- Morton HE. The relationship of concentration and germicidal efficiency of ethyl alcohol. Ann NY Acad Sci. 1950 Aug;53:191-6.
- Tilley FW. An experimental study of the influence of temperature on the bactericidal activities of alcohols and phenols. J Bacteriol. 1942 Apr;43(4):521-5.
- Kampf G, Rudolf M, Labadie JC, Barrett S. Spectrum of antimicrobial activity and user acceptability of the hand disinfectant agent Sterillium Gel. J Hosp Infect. 2002 Oct;52(2):141-7.
- Adams D, Quayam M, Worthington T, Lambert P, Elliott T. Evaluation of a 2% chlorhexidine gluconate in 70% isopropyl alcohol skin disinfectant. J Hosp Infect. 2005;61:287-90.
- Kampf G, Hollingsworth A. Comprehensive bactericidal activity of an ethanol-based hand gel in 15 seconds. Ann Clin Microbiol Antimicrob. 2008 Jan 22;7:2.
- Kampf G, Hollingsworth A. Validity of the four European test strains of prEN 12054 for the determination of comprehensive bactericidal activity of an alcohol-based hand rub. J Hosp Infect. 2003;55:226-31.
- Kampf G, Meyer B, Goroncy-Bermes P. Comparison of two test methods for the determination of sufficient antimicrobial efficacy of three different alcohol-based hand rubs for hygienic hand disinfection. J Hosp Infect. 2003;55:220-5.
- Kampf G, Höfer M, Wendt C. Efficacy of hand disinfectants against vancomycin-resistant enterococci in vitro. J Hosp Infect. 1999;42:143-50.
- Akamatsu T, Tabata K, Hironga M, Kawakami H, Uyeda M. Transmission of Helicobacter pylori infection via flexible fiberoptic endoscopy. Am J Infect Control. 1996 Oct;24(5):396-401.
- Rutala WA, Barbee SL, Aguiar NC, Sobsey MD, Weber DJ. Antimicrobial activity of home disinfectants and natural products against potential human pathogens. Infect Control Hosp Epidemiol. 2000 Jan;21(1):33-8.
- Smith CR. Alcohol as a disinfectant against tubercle bacillus. Public Health Rep. 1947 Sep 5;62(36):1285-95.
- Best M, Sattar SA, Springthorpe VS, Kennedy ME. Comparative mycobactericidal efficacy of chemical disinfectants in suspension and carrier tests. Appl Environ Microbiol. 1988 Nov;54(11):2856-8.
- Best M, Sattar SA, Springthorpe VS, Kennedy ME. Efficacies of selected disinfectants against Mycobacterium tuberculosis. J Clin Microbiol. 1990 Oct;28(10):2234-9.
- Kruse RH, Green TD, Chambers BC, Jones MW. Disinfection of aerosolized pathogenic fungi on laboratory surfaces. II. Culture phase. Appl Microbiol. 1964 Mar;12(2):115-60.
- van Engelenburg FAC, Terpstra FG, Schuitemaker H, Moorer WR. The virucidal spectrum of a high concentration alcohol mixture. J Hosp Infect. 2002 Jun;51(2):121-5.
- Woolwine JD, Gerberding JL. Effect of testing method on apparent activities of antiviral disinfectants and antiseptics. Antimicrob Agents Chemother. 1995 Apr;39(4):921–3.
- Martin LS, Meoougal JS, Loskoski SL. Disinfection and inactivation of the human T lymphotropic virus type III/lymphadenopathy associated virus. J Infect Dis. 1985 Aug;152(2):400-3.
- Groupe V, Engle CC, Gaffney PE. Virucidal activity of representative antiinfective agents against influenza A and vaccinia virus. Appl Microbiol. 1955 Nov;3(6):333-6.
- Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions, and chemical reagents. Jpn J Vet Res. 2004;52(3):105-12.
- Bond WXV, Favero MS, Petersen N Jr. Inactivation of hepatitis B virus by intermediate to high-level disinfectant chemicals. J Clin Microbiol. 1983 Sep;18(3):535-8.
- Kobayashi H, Tsuzuki M, Koshimizu K. Susceptibility of hepatitis B virus to disinfectants or heat. J Clin Microbiol. 1984 Aug;20(2):214-6.
- Sattar SA, Tetro J, Springthorpe VS, Giulivi A. Preventing the spread of hepatitis B and C viruses: where are germicides relevant? Am J Infect Control. 2001 Jun;29(3):187-97.
- Wolff MH, Schmitt J, Rahaus M, König A. Hepatitis A virus: a test method for virucidal activity. J Hosp Infect. 2001 Aug;48 Suppl A:S18-22.
- Klein M, Deforest A. Antiviral action of germicides. Soap Chem Spec. 1963;39:70-2.
- Kurtz JB. Virucidal effects of alcohols against echovirus 11. Lancet. 1979 Mar 3;1(8114):496-7.
- Spire B, Barre-Sinoussi F, Montagnier L. Inactivation of lymphadenopathy associated virus by chemical disinfectants. Lancet. 1984;ii:899-901.
- Kampf G, Steinmann J, Rabenau H. Suitability of vaccinia virus and bovine viral diarrhea virus (BVDV) for determining activities of three commonly-used alcohol-based hand rubs against enveloped viruses. BMC Infect Dis. 2007 Feb 9;7:5.
- Trujillo R, N Laible. Reversible inhibition of spore germination by alcohols. Appl Microbiol. 1970 Oct;20(4):620-3.
- Hare R, Raik E, Gash S. Efficiency of antiseptics when acting on dried organisms. Br Med J. 1963 Feb 23;1(5329):496-500.
- Barbee SL, Weber DJ, Sobsey MD, Rutala WA. Inactivation of Cryptosporidium parvum oocyst infectivity by disinfection and sterilization processes. Gastrointest Endosc. 1999 May;49(5):605-11.
- Gershenfeld L. The sterility of alcohol. Am J Med Sci. 1938;195:358-61.
- Harrington C, Walker H. The germicidal action of alcohol. Boston Med Surg J. 1903;148:548-52.
- Tainter ML, Throndson AU, Beard RB. Chemical sterilization of instruments. J Am Dent Assoc. 1944;31:479-89.
- Rutala WA, Weber DJ. Selection and use of disinfectants in healthcare. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control, 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins. 2004:1473-522.
- Marples RR, Towers AG. A laboratory model for the investigation of contact transfer of microorganisms. J Hyg (Camb). 1979;82:237-48.
- Eckert RN, Ehrenkranz NJ, Alfonso BC. Indications for alcohol or bland soap removal of aerobic Gram-negativce skin bacteria: assessment by a novel method. Infect Control Hosp Epidemiol. 1989;10:306-11.
- Lilly HA, Lowbury EJL. Transient skin flora. Their removal by cleansing or disinfection in relation to their mode of deposition. J Clin Pathol. 1978;31:919-22.
- Mbithi JN, Springthorpe VS, Sattar SA. Comparative in vivo efficiencies of hand washing agents against hepatitis A virus (HM-175) and poliovirus type 1 (Sabin). Appl Environ Microbiol. 1993 Oct;59(10):3463-9.
- Sattar SA, Abebe M, Bueti AJ, et al. Activity of an alcohol-based hand gel against human adeno-, rhino-, and rotaviruses using the fingerpad method. Infect Control Hosp Epidemiol. 2000 Aug;21(8):516-9.
- Gehrke C, Steinmann J, Goroncy-Bermes P. Inactivation of feline calicivirus, a surrogate of norovirus (formerly Norwalk-like viruses), by different types of alcohol in vitro and in vivo. J Hosp Infect. 2004;56:49-55.
- Ansari SA, Sattar SA, Springthorpe VS, Wells GA, Tostawaryk W. In vivo protocol for testing efficacy of hand-washing agents against viruses and bacteria: experiments with rotavirus and Escherichia coli. Rev Infect Dis. 1989 Dec;55(12):3113-8.
- Schurmann W, Eggers HJ. Antiviral activity of an alcoholic hand disinfectant: comparison of the in vitro suspension test with in vivo experiments on hands, and on individual fingertips. Antiviral Res. 1983 Mar;3(1):25-41.
- Ayliffe GAJ. Surgical scrub and skin disinfection. Infect Control. 1984;5(1):23-7.
- Sickbert-Bennett EE, Weber DJ, Gergen-Teague MF, Sobsey MD, Samsa GP, Rutala WA. Comparative efficacy of hand hygiene agents in the reduction of bacteria and viruses. Am J Infect Control. 2005;33:67-77.
- Dharan S, Hugonnet S, Sax H, Pittet D. Comparison of waterless hand antisepsis agents at short application times: raising the flag of concern. Infect Control Hosp Epidemiol. 2003 Mar;24(3):160-4.
- Weber DJ, Sickbert-Bennett E, Gergen MF, Rutala WA. Efficacy of selected hand hygiene agents used to remove Bacillus atrophaeus (a surrogate of Bacillus anthracis) from contaminated hands. JAMA. 2003 Mar 12;289(10):1274-7.
- Dyer DL, Gerenraich KB, Wadhams PS. Testing a new alcohol-free hand sanitizer to combat infection. AORN J. 1998 Aug;68:239-51.
- Moadab A, Rupley KF, Wadhams P. Effectiveness of a nonrinse, alcohol-free antiseptic handwash. J Am Podiatr Med Assoc. 2001 Jun;91(6):288-93.
- Jones DL, Jampani H, Mulberry G, Rizer RL. Moisturizing alcohol gels for surgical hand preparation. AORN J. 2000 Mar;71(3):589-99.
- Rotter ML. Arguments for alcoholic hand disinfection. J Hosp Infect. 2001;48(Suppl A):S4-S8.
- Ojajärvi J. Effectiveness of hand washing and disinfection methods in removing transient bacteria after patient nursing. J Hyg (Lond). 1980 Oct;85(2):193-203.
- Cardoso CL, Pereira HH, Zequim JC, Guilhermetti M. Effectiveness of hand-cleansing agents for removing Acinetobacter baumannii strain from contaminated hands. Am J Infect Control. 1999;27:327-31.
- Guilhermetti M, Hernandes SED, Fukushigue Y, Garcia LB, Cardoso CL. Effectiveness of hand-cleansing agents for removing methicillin-resistant Staphylococcus aureus from contaminated hands. Infect Control Hosp Epidemiol. 2001 Feb;22(2):105-8.
- Ayliffe GAJ, Babb JR, Quoraishi AH. A test for ‘hygienic’ hand disinfection. J Clin Pathol. 1978;31:923-8.
- Kampf G, Shaffer M, Hunte C. Insufficient neutralization in testing a chlorhexidine-containing ethanol-based hand rub can result in false positive efficacy assessment. BMC Infect Dis. 2005 Jun 20;5(1):48.
- Kramer A, Rudolph P, Kampf G, Pittet D. Limited efficacy of alcohol-based hand gels. Lancet. 2002 Apr 27;359(9316):1489-90.
- Peitsch H. Hand antiseptics: rubs versus scrubs, alcoholic solutions versus alcoholic gels. J Hosp Infect. 2001;48 Suppl A:S33-6.
- Herruzo R, Vizcaino MJ, Herruzo I. In vitro–in vivo sequence studies as a method of selecting the most efficacious alcohol-based solution for hygienic hand disinfection. Clin Microbial Infect. 2010 May;16:518-23.
- Kampf G, Ostermeyer C. Inter-laboratory reproducibility of the EN 1500 reference hand disinfection. J Hosp Infect. 2003 Apr;53:304-6.
- Kampf G, Ostermeyer C. Intra-laboratory reproducibility of the hand hygiene reference procedures of EN 1499 (hygienic hand wash) and EN 1500 (hygienic hand disinfection). J Hosp Infect. 2002 Nov;52(3):219-24.
- Rotter ML, Koller W, Wewalka G, Werner HP, Ayliffe GAJ, Babb JR. Evaluation of procedures for hygienic hand disinfection: controlled parallel experiments on the Vienna test model. J Hyg (Lond). 1986 Feb;96(1):27-37.
- Lowbury EJL, Lilly HA, Ayliffe GAJ. Preoperative disinfection of surgeons’ hands: use of alcoholic solutions and effects of gloves on skin flora. Br Med J. 1974;4:369-72.
- Lilly HA, Lowbury EJL, Wilkins MD. Limits to progressive reduction of resident skin bacteria by disinfection. J Clin Pathol. 1979;32:382-5.
- Kampf G, Ostermeyer C. Efficacy of two distinct ethanol-based hand rubs for surgical hand disinfection – a controlled trial according to prEN 12791. BMC Infect Dis. 2005 Mar 22;5(1):17.
- Lowbury EJL, Lilly HA. Disinfection of the hands of surgeons and nurses. Br Med J. 1960;1:1445-50.
- Nhung DTT, Freydiere AM, Constant H, Falson F, Pirot F. Sustained antibacterial effect of a hand rub gel incorporating chlorhexidine-loaded nanocapsules (Nanochlorex). Int J Pharm. 2007 Apr 4;334(1-2):166-72.
- Lilly HA, Lowbury EJL, Wilkins MD. Detergents compared with each other and with antiseptics as skin ‘degerming’ agents. J Hyg (Lond). 1979;82:89-93.
- Kampf G, Kapella M. Suitability of Sterillium Gel for surgical hand disinfection. J Hosp Infect. 2003 Jul;54(3):222-5.
- Morrison AJ, Gratz J, Cabezudo I, Wenzel RP. The efficacy of several new handwashing agents for removing non-transient bacterial flora from hands. Infect Control. 1986;7(5):268-72.
- Larson EL, Eke PI, Laughon BE. Efficacy of alcohol-based hand rinses under frequent-use conditions. Antimicrob Agents Chemother. 1986 Oct;30(4):542-4.
- Larson EL, Eke PI, Wilder MP, Laughon BE. Quantity of soap as a variable in handwashing. Infect Control. 1987 Sep;8(9):371-5.
- Babb JR, Davies JG, Ayliffe GAJ. A test procedure for evaluating surgical hand disinfection. J Hosp Infect. 1991;18(Suppl B):41-9.
- Rotter ML, Simpson RA, Koller W. Surgical hand disinfection with alcohols at various concentrations: parallel experiments using the new proposed European standards method. Infect Control Hosp Epidemiol. 1998;19:778-81.
- Rotter ML, Kampf G, Suchomel M, Kundi M. Population kinetics of the skin flora on gloved hands following surgical hand disinfection with 3 propanol-based hand rubs: a prospective, randomized, double-blind trial. Infect Control Hosp Epidemiol. 2007 Mar;28(3):346-50.
- Aly R, Maibach HI. Comparative study on the antimicrobial effect of 0.5% chlorhexidine gluconate and 70% isopropyl alcohol on the normal flora of hands. Appl Environ Microbiol. 1979 Mar;37(3):610–3.
- Rotter M, Koller W, Wewalka G. Povidone-iodine and chlorhexidine gluconate detergents for disinfection of the hands. J Hosp Infect. 1980;1:149-58.
- Larson EL, Butz AM, Gullette DL, Laughon BA. Alcohol for surgical scrubbing? Infect Control Hosp Epidemiol. 1990;11:139-43.
- Paulson DS, Fendler EJ, Dolan MJ, Williams RA. A close look at alcohol gel as an antimicrobial sanitizing agent. Am J Infect Control. 1999 Aug;27(4):332-8.
- Goroncy-Bermes P. Hand disinfection according to the European standard EN 1500 (hygienic hand rub): a study with Gram-negative and Gram-positive test organisms. Int J Hyg Environ Health. 2001;204(2-3):123-6.
- Mulberry G, Snyder AT, Heilman J, Pyrek J, Stahl J. Evaluation of a waterless, scrubless chlorhexidine gluconate/ethanol surgical scrub for antimicrobial efficacy. Am J Infect Control. 2001;29:377-82.
- Seal LA, Rizer RL, Maas-Irslinger R. A unique water optional health care personnel handwash provides antimicrobial perisistence and residual effects while decreasing the need for additional products. Am J Infect Control. 2005;33:207-16.
- Suchomel M, Gnant G, Wienlich M, Rotter M. Surgical hand disinfection using alcohol: the effects of alcohol type, mode and duration of application. J Hosp Infect. 2009;71:228-33.
- Centers for Disease Control and Prevention. Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR. 2003 Jun 6;52(No. RR-10). 42 p.
- Rutala WA, Weber DJ; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for disinfection and sterilization in healthcare facilities, 2008. Atlanta (GA): Centers for Disease Control and Prevention. 2008. 158 p.
- Kruse RH, Green TD, Chambers RC, Jones MW. Disinfection of aerosolized pathogenic fungi on laboratory surfaces. I. Tissue phase. Appl Microbiol. 1963;11:436-45.
- Frobisher M, Sommermeyer L, Blackwell MJ. Studies on disinfection of clinical thermometers. I. Oral thermometers. Appl Microbiol. 1973 Jul;1(4):187-94.
- Wright ES, Mundy RA. Studies on disinfection of clinical thermometers. I. Oral thermometers from a general hospital. Appl Microbiol. 1958 Nov;6(6):381-3.
- Wright ES, Mundy RA. Studies on disinfection of clinical thermometers. II. Oral thermometers from a tuberculosis sanatorium. Appl Microbiol. 1961 Nov;9:508-10.
- Sommermeyer L, Frobisher M. Laboratory studies on disinfection of rectal thermometers. Nurs Res. 1953 Oct;2(2):85-9.
- Spach DH, Silverstein FE, Stamm WE. Transmission of infection by gastrointestinal endoscopy and bronchoscopy. Ann Intern Medicine. 1993 Jan 15;118(2):117-28.
- Struelens MJ, Rost F, Deplano A, Maas A, Schwam V, Serruys E, Cremer M. Pseudomonas aeroginosa and Enterobacteriaceae bacteremia after biliary endoscopy: an outbreak investigation using DNA macrorestriction analysis. Am J Med. 1993 Nov;95(5):489-98.
- Birnie GG, Quigley EM, Clements GB, Follet EA, Watkinson G. Endoscopic transmission of hepatitis B virus. Gut. 1983 Feb;24(2):171-4.
- Michele TM, Cronin WA, Graham NM, Dwyer DM, Pope DS, Harrington S, Chaisson RE, Bishai WR. Transmission of Mycobacterium tuberculosis by fiberoptic bronchoscope: identification by DNA fingerprinting. JAMA. 1997 Oct 1;278(13):1093-5.
- Langenberg W, Rauws EA, Oudbier JH, Tytgat GN. Patient-to-patient transmission of Campylobacter pylori infection by fiberoptic gastroduodenoscopy and biopsy. J Infect Dis. 1990 Mar;161(3):507-11.
- US Food and Drug Administration, CDC. Public health advisory: infections from endoscopes inadequately reprocessed by an automated endoscope reprocessing system. September 10, 1999. Available at http://www.fda.gov/cdrh/safety/endoreprocess.pdf.
- Garcia de Cabo A, Martinez Larriba PL, Checa Pinilla J, Guerra Sanz F. A new method of disinfection of the flexible fibrebronchoscope. Thorax. 1978 Apr;33(2):270-2.
- Alvarado CJ, Reichelderfer M. APIC guideline for infection prevention and control in flexible endoscopy. Am J Infect Control. 2000 Apr;28(2):138-55.
- Rutala WA, Clontz EP, Weber DJ, Hoffmann KK. Disinfection practices for endoscopes and other semicritical items. Infect Control Hosp Epidemiol. 1991 May;12(5):282-8.
- Beck-Sague CM, Jarvis WR. Epidemic bloodstream infections associated with pressure transducers: a persistent problem. Infect Control Hosp Epidemiol. 1989 Feb;10(2):54-9.
- Koo D, Bouvier B, Wesley M, Courtright P, Reingold A. Epidemic keratoconjunctivitis in a university medical center ophthalmology clinic; need for re-evaluation of the design and disinfection of instruments. Infect Control Hosp Epidemiol. 1989 Dec;10(12):547-52.
- Talbot GH, Skros M, Provencher M. 70% alcohol disinfection of transducer heads: experimental trials. Infect Control. 1985 Jun;6(6):237-9.
- Platt R, Lehr JL, Marino S, Munoz A, Nash B, Raemer DB. Safe and cost-effective cleaning of pressure-monitoring transducers. Infect Control Hosp Epidemiol. 1988 Sep;9(9):409-16.
- Pepose JS, Linette G, Lee SF, MacRae S. Disinfection of Goldmann tonometers against human immunodeficiency virus type 1. Arch Ophthalmol. 1989 Jul;107(7):983-5.
- Sattar SA, Springthorpe VS, Karim Y, Loro P. Chemical disinfection of non-porous inanimate surfaces experimentally contaminated with four human pathogenic viruses. Epidemiol Infect. 1989 Jun;102(3):493-505.
- Rutala WA, Peacock JE, Gergen MF, Sobsey MD, Weber DJ. Efficacy of hospital germicides against adenovirus 8, a common cause of epidemic keratoconjunctivitis in health care facilities. Antimicrob Agents Chemother. 2006 Apr;50(4):1419-24.
- Jernigan JA, Lowry BS, Hayden FG, Kyger SA, Conway BP, Gröschel DHM, Farr BM. Adenovirus type 8 epidemic keratoconjunctivitis in an eye clinic: risk factors and control. J Infect Dis. 1993 Jun;167(6):1307-13.
- Singh D, Kaur H, Gardner WG, Treen LB. Bacterial contamination of hospital pagers. Infect Control Hosp Epidemiol. 2002 May;23(5):274-6.
- Ohara T, Itoh Y, Itoh K. Ultrasound instruments as possible vectors of staphylococcal infection. J Hosp Infect. 1998 Sep;40(1):73-7.
- Embil JM, Zhanel GG, Plourde J, Hoban D. Scissors: a potential source of nosocomial infection. Infect Control Hosp Epidemiol. 2002 Mar;23(3):560-4.
- Bernard L, Kereveur A, Durand D, Gonot J, Goldstein F, Mainardi JL, Acar J, Carlet J. Bacterial contamination of hospital physician’s stethoscopes. Infect Control Hosp Epidemiol. 1999 Sep;20(9):626-8.
- Cavagnolo RZ. Inactivation of herpesvirus on CPR manikins utilizing a currently recommended disinfecting procedure. Infect Control. 1985 Nov;6(11):456-8.
- Lilly HA, Lowbury EJL, Wilkins MD, Zaggy A. Delayed antimicrobial effects of skin disinfection by alcohol. J Hyg (Lond). 1979;82:497-500.
- Chlorhexidine Gluconate Misc. In: McEvoy GK, Snow EK, Miller J, Kester L, Welsh OH Jr, Heydorn JD, Le T, Mendham NA, O’Rourke A, Braun S, Grande KJ, Litvak K, editors. AHFS Drug Information [Internet]. Bethesda: American Society of Health-System Pharmacists, Inc. c2010; [cited 2010 Oct 9]; [about 4 screens]. Available from http://www.medscape.com/druginfo/monograph?cid=med&drugid=4264&drugname=Chlorhexidine+
Gluconate+Misc&monotype=monograph&secid=4. Subscription required to view. - Kampf G. What is left to justify the use of chlorhexidine in hand hygiene? J Hosp Infect. 2008 Oct;70 Suppl 1:27-34.
- Cousido MC, Tomás M, Tomás I, et al. Effect of a neutralizing agent on the evaluation of the antimicrobial activity of chlorhexidine on the bacterial salivary flora. Arch Oral Biol. 2008;53:981-4.
- Kampf G, Shaffer M, Hunte C. Insufficient neutralization in testing a chlorhexidine-containing ethanol-based hand rub can result in false positive efficacy assessment. BMC Infect Dis. 2005 Jun 20;5(1):48.
- Reichel M, Heisig P, Kampf G. Pitfalls in efficacy testing – how important is the validation of neutralization of chlorhexidine digluconate? Ann Clin Microbiol Antimicrob. 2008;7:20.
- Hibbard JS. Analyses comparing the antimicrobial activity and safety of current antiseptic agents: a review. J Infus Nurs. 2005 May-Jun;28(3):194-207.
- Hibbard JS, Mulberry GK, Brady AR. A clinical study comparing the skin antisepsis and safety of ChloraPrep, 70% isopropyl alcohol, and 2% aqueous chlorhexidine. J Infus Nurs. 2002 Jul-Aug;25(4):244-9.
- Hibbard JS. Administration of 2% chlorhexidine gluconate in 70% isopropyl alcohol is effective in 30 seconds. Infect Control Hosp Epidemiol. 2002 May;23(5):233-4.
- Reichel M, Heisig P, Kohlmann T, Kampf G. Alcohols for skin antisepsis at clinically relevant skin sites. Antimicrob Agents Chemother. 2009 Nov;53(11):4778-82.
- Eckert DG, Ehrenkranz NJ, Alfonso BC, Moskowitz LB. Proteeae groin skin carriage among nursing home residents—resistance to antiseptics. Infect Control Hosp Epidemiol. 1989 Apr;10(4):155-60.
- Pittet D, Dharan S, Touveneau S, Sauvan V, Perneger TV. Bacterial contamination of the hands of hospital staff during routine patient care. Arch Intern Med. 1999;159:821-6.
- Pessoa-Silva CL, Dharan S, Hugonnet S, Touveneau S, Posfay-Barbe K, Pfister R, Pittett D. Dynamics of bacterial hand contamination during routine neonatal care. Infect Control Hosp Epidemiol. 2004 May;25(3):192-7.
- Casewell M, Phillips I. Hands as route of transmission for Klebsiella Br Med J. 1977;2:1315-7.
- Ehrenkranz NJ, Alfonso BC. Failure of bland soap handwash to prevent hand transfer of patient bacteria to urethral catheters. Infect Control Hosp Epidemiol. 1991;12:654-62.
- Larson EL, Norton Hughes CA, Pyrak JD, Sparks SM, Cagatay EU, Bartkus JM. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control. 1998;26:513–21.
- Wade JJ, Casewell MW. The evaluation of residual antimicrobial activity on hands and its clinical relevance. J Hosp Infect. 1991 Jun;18 Suppl B;23-8.
- McDonald LC. Hand hygiene in the new millennium: drawing the distinction between efficacy and effectiveness. Infect Control Hosp Epidemiol. 2003 Mar;24(3):157-9.
- Larson E. A causal link between handwashing and risk of infection? Examination of the evidence. Infect Control Hosp Epidemiol. 1988;9(1):28-36.
- Prombo MP, Tilden EB. Evaluation of disinfectants by tests in vivo. J Dent Res. 1950:29(2):108-22.
- Kac G, Masmejean E, Gueneret M, Rodi A, Peyrard S, Podglajen I. Bactericidal efficacy of a 1.5 min surgical hand-rubbing protocol under in-use conditions. J Hosp Infect. 2009;72:135-9.
- Bryce EA, Spence D, Roberts FJ. An in-use evaluation of an alcohol-based pre-surgical hand disinfectant. Infect Control Hosp Epidemiol. 2001 Oct;22(10):635-9.
- Larson EL, Aiello A, Heilman JM, Lyle CT, Cronquist A, Stahl JB, Della-Latta P. Comparison of different regimens for surgical hand preparation. AORN J. 2001 Feb;73(2):412-20.
- Berman M. One hospital’s clinical evaluation of brushless scrubbing. AORN J. 2004 Feb;79(2):349-58.
- Parienti JJ, Thibon P, Heller R, Le Roux Y, von Theobald P, Bensadoun H, Bouvet A, Lemarchand F, Coutour X. Hand-rubbing with an aqueous alcoholic solution vs traditional surgical hand-scrubbing and 30-day surgical site infection rates. JAMA. 2002 Aug 14;288(6):722-7.
- Meadows E, Le Saux N. A systematic review of the effectiveness of antimicrobial rinse-free hand sanitizers for prevention of illness-related absenteeism in elementary school children. BMC Public Health. 2004 Nov 1;4:50.
- Mody L, McNeil SA, Sun R, Bradley SE, Kauffman CA. Introduction of a waterless alcohol-based hand rub in a long-term facility. Infect Control Hosp Epidemiol. 2003 Mar;24(3):165-71.
- Tvedt C, Bukholm G. Alcohol-based hand disinfection: a more robust hand-hygiene method in an intensive care unit. J Hosp Infect. 2005 Mar;59(3):229-34.
- Brown SM, Lubimova AV, Khrustalyeva NM, Shulaeva SV, Tekhova I, Zueva LP, Goldmann D, O’Rourke EJ. Use of an alcohol-based hand rub and quality improvement interventions to improve hand hygiene in a Russian neonatal intensive care unit. Infect Control Hosp Epidemiol. 2003 Mar;24(3):172-9.
- McNeil SA, Foster CL, Hedderwick SA, Kauffman CA. Effect of hand cleansing with antimicrobial soap or alcohol-based gel on microbial colonization of artificial fingernails worn by healthcare workers. Clin Infect Dis. 2001 Feb 1;32(3):367-72.
- Trick WE, Vernon MO, Hayes RA, Nathan C, Rice TW, Peterson BJ, Segreti J, Welbel SF, Solomon SL, Weinstein RA. Impact of ring wearing on hand contamination and comparison of hand hygiene agents in a hospital. Clin Infect Dis. 2003 Jun 1;36(11):1383-90.
- Zaragoza M, Sallés M, Gomez J, Bayas JM, Trilla A. Handwashing with soap or alcoholic solutions? A randomized clinical trial of its effectiveness. Am J Infect Control. 1999 Jun;27(3):258-61.
- Lucet JC, Rigaud MP, Mentre F, Kassis N, Deblangy C, Andremont A, Bouvet E. Hand contamination before and after different hand hygiene techniques: a randomized clinical trial. J Hosp Infect. 2002;50:276-80.
- Kac G, Podglajen I, Gueneret M, Vaupré S, Bissery A, Meyer G. Microbiological evaluation of two hand hygiene procedures achieved by healthcare workers during routine patient care: a randomized study. J Hosp Infect. 2005 May;60(1):32-9.
- Barbut F, Maury E, Goldwirt L, Boëlle PY, Neyme D, Aman R, Rossi B, Offenstadt G. Comparison of the antibacterial efficacy and acceptability of an alcohol-based hand rinse with two alcohol-based hand gels during routine patient care. J Hosp Infect. 2007 Jun;66(2):167-73.
- Winnefeld M, Richard MA, Drancourt M, Grob JJ. Skin tolerance and effectiveness of two hand decontamination procedures in everyday hospital use. Br J Dermatol. 2000 Sep;143(3):546-50.
- Widmer AE, Dangel M. Alcohol-based handrub: evaluation of technique and microbiological efficacy with international infection control professionals. Infect Control Hosp Epidemiol. 2004 Mar;25(3):207-9.
- Larson E, Silberger M, Jakob K, Whittier S, Lai L, Della-Latta P, Saiman L. Assessment of alternative hand hygiene regimens to improve skin health among neonatal intensive care unit nurses. Heart Lung. 2000 Mar-Apr;29(2):136-42.
- Malani A, Trimble K, Parekh V, Chenoweth C, Kaufman S, Saint S. Review of clinical trials of skin antiseptic agents used to reduce blood culture contamination. Infect Control Hosp Epidemiol. 2007 Jul;28(7):892-5.
- Mackintosh CA, Hoffman PN. An extended model for transfer of micro-organisms via the hands: differences between organisms and the effect of alcoholic disinfectants. J Hyg (Lond). 1984 Jun;92(3):345-55.
- Girou E, Loyeau S, Legrand P, Oppein F, Brun-Buisson. Efficacy of handrubbing with alcohol based solution versus standard handwashing with antiseptic soap: randomized clinical trial. Br Med J. 2002 Aug 17;325(7360):362-6.
- Rosenthal VD, Guzman S, Safdar N. Reduction in nosocomial infection with improved hand hygiene in intensive care units of a tertiary care hospital in Argentina. Am J Infect Control. 2005 Sep;33(7):392-7.
- Lam BC, Lee J, Lau YL. Hand hygiene practices in a neonatal intensive care unit: a multimodal intervention and impact on nosocomial infection. Pediatrics. 2004 Nov;114(5):e565-71.
- Johnson PDR, Martin R, Burrell LJ, Grabsch EA, Kirsa SW, O’Keefe J, Mayall BC, Edmonds D, Barr W, Bolger C, Naidoo H, Grayson ML. Efficacy of an alcohol/chlorhexidine hand hygiene program in a hospital with high rates of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection. MJA. 2005;183:509-14.
- Knight N, Strait T, Anthony N, et al. Clostridium difficile colitis: a retrospective study of incidence and severity before and after institution of an alcohol-based hand rub policy. Am J Infect Control. 2010 Sep;38(7):523-8.
- Larson EL, Cimiotti J, Haas J, Parides M, Nesin M, Della-Latta P, Saiman L. Effect of antiseptic handwashing vs alcohol sanitizer on health care-associated infections in neonatal intensive care units. Arch Pediatr Adolesc Med. 2005 Apr;159:377-83.
- Doebbeling BN, Stanley GL, Sheetz CT, Pfaller MA, Houston AK, Annis L, Li N, Wenzel RP. Comparative efficacy of alternative hand-washing agents in reducing nosocomial infections in intensive care units. N Engl J Med. 1992 Jul 9;327(2):88-93.
- Aragorn D, Sole ML, Brown S. Outcomes of an infection prevention project focusing on hand hygiene and isolation practices. AACN Clin Issues. 2005 Apr-Jun;16(2):121-32.
- Rupp ME, Fitzgerald T, Puumala S, Anderson JR, Craig R, Iwen PC, Jourdan D, Keuchel J, Marion N, Peterson D, Sholtz L, Smith V. Prospective, controlled, cross-over trial of alcohol-based hand gel in critical care units. Infect Control Hosp Epidemiol. 2008 Jan;29(1):8-15.
- Nguyen KV, Nguyen PT, Jones SL. Effectiveness of an alcohol-based hand hygiene programme in reducing nosocomial infections in the Urology Ward of Binh Dan Hospital, Vietnam. Trop Med Int Health. 2008 Oct;13(10):1297-302.
- Le TA, Dibley MJ, Vo VN, et al. Reduction in surgical site infection in neurosurgical patients associated with a bedside hand hygiene program in Vietnam. Infect Control Hosp Epidemiol. 2007 May;28(5):583-8.
- Herud T, Nilsen RM, Svendheim K, Harthug S. Association between use of hand hygiene products and rates of healthcare-associated infections in a large university hospital in Norway. Am J Infect Control. 2009 May;37(4):311-7.
- Neuzil KM, Hohlbein C, Zhu Y. Illness among schoolchildren during influenza season: effect on school absenteeism, parental absenteeism from work, and secondary illness in families. Arch Pediatr Adolesc Med. 2002 Oct;156(10):986-91.
- Guinan ME, McGuckin-Guinan M, Sevareid A. Who washes hands after using the bathroom? Am J Infect Control. 1997 Oct;25(5):424-5.
- Morton JL, Schultz AA. Healthy hands: use of alcohol gel as an adjunct to handwashing in elementary school children. J Sch Nurs. 2004 Jun;20(3):161-7.
- Thompson K. The effects of alcohol hand sanitizer on elementary school absences. Am J Infect Control. 2004 May;32(3):E127.
- Hammond B, Ali Y, Fendler E, Dolan M, Donovan S. Effect of hand sanitizer on elementary school absenteeism. Am J Infect Control. 2000 Oct;28(5):340-6.
- Guinan M, McGuckin M, Ali Y. The effect of a comprehensive handwashing program on absenteeism in elementary schools. Am J Infect Control. 2002 Jun;30(4):217-20.
- Vessey JA, Sherwood JJ, Warner D, Clark D. Comparing hand washing to hand sanitizers in reducing elementary school students’ absenteeism. Pediatr Nurs. 2007 Jul-Aug;33(4):368-72.
- Sandora TJ, Shih MC, Goldmann DA. Reducing absenteeism from gastrointestinal and respiratory illness in elementary students: a randomized, controlled trial of an infection-control intervention. Pediatrics. 2008 Jun;121(6):e1555-62.
- Embil JM, Dyck B, Plourde P. Prevention and control of infections in the home. CMAJ. 2009 May 26;180(11):E82-6.
- Widmer AF, Conzelmann M, Tomic M, Frei R, Stranden AM. Introducing alcohol-based hand rub for hand hygiene: the critical need for training. Infect Control Hosp Epidemiol. 2007 Jan;28(1):50-4.
- Kampf G, Ostermeyer C. Efficacy of alcohol-based hand gels compared with simple hand wash and hygienic hand disinfection. J Hosp Infect. 2004 Apr;56(Suppl 2):13-5.
- Hseuh PR, Teng LJ, Yang PC, Pan HL, Ho SW, Luh KT. Nosocomial pseudoepidemic caused by Bacillus cereus traced to contaminated ethyl alcohol from a liquor factory. J Clin Microbiol. 1999 Jul;37(7):2280-4.
- Nasser RM, Rahi AC, Haddad MF, Daoud Z, Irani-Hakime N, Almawi WY. Outbreak of Bulkholderia cepacia bacteremia traced to contaminated hospital water used for dilution of an alcohol skin antiseptic. Infect Control Hosp Epidemiol. 2004 Mar;25(3):231-9.
- Widmer AF. Replace hand washing with use of a waterless alcohol hand rub? Clin Infect Dis. 2000 Jul;31(1):136-43.
- Ghannoum M, O’Toole GA, editors. Microbial Biofilms. Herndon (VA): ASM Press. 2004:250-68.
- Smith K, Hunter IS. Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J Med Microbiol. 2008;57:966-73.
- Potera C. Forging a link between biofilms and disease. Science. 1999;283:1837-8.
- Toté K, Horemans T, Vanden Berghe D, Maes L, Cos P. Inhibitory effect of biocides on the viable masses and matrices of Staphylococcus aureus and Pseudomonas aeruginosa Appl Environ Microbiol. 2010 May;76(10):3135-42.
- Adams D, Quayam M, Worthington T, Lambert P, Elliott T. Evaluation of a 2% chlorhexidine gluconate in 70% isopropyl alcohol skin disinfectant. J Hosp Infect. 2005;61:287-90.
- Wong HS, Townsend KM, Fenwick SG, Trengove RD, O’Handley RM. Comparative susceptibility of planktonic and 3-day-old Salmonella typhimurium biofilms to disinfectants. J Appl Microbiol. 2010;108:2222-8.
- Nett JE, Guite KM, Ringeisen A, Holoyda KA, Andes DR. Reduced biocide susceptibility in Candida albicans Antimicrob Agents Chemother. 2008 Sep;52(9):3411-3.
- Boyce JM. Using alcohol for hand antisepsis: dispelling old myths. Infect Control Hosp Epidemiol. 2000 Jul;21(7):438-40.
- Löffler H, Kampf G. Hand disinfection: how irritant are alcohols? J Hosp Infect. 2008;70(Suppl 1):44-8.
- Boyce JM, Kelliher S, Vallande N. Skin irritation and dryness associated with two hand-hygiene regimens: soap-and-water hand washing versus hand antisepsis with an alcoholic hand gel. Infect Control Hosp Epidemiol. 2000 Jul;21:442-48.
- Pereira LJ, Lee GM, Wade KJ. An evaluation of five protocols for surgical handwashing in relation to skin condition and microbial counts. J Hosp Infect. 1997;36:49-65.
- Larson EL. Hygiene of the skin: when is clean too clean? Emerg Infect Dis. 2001 Mar-Apr;7(2):225-8.
- Prevention, Pesticides and Toxic Substances. Reregistration Eligibility Decision (RED): Aliphatic Alcohols. EPA-738-R-95-013. Washington, DC: United States Environmental Protection Agency. 1995 Apr. 238 p.



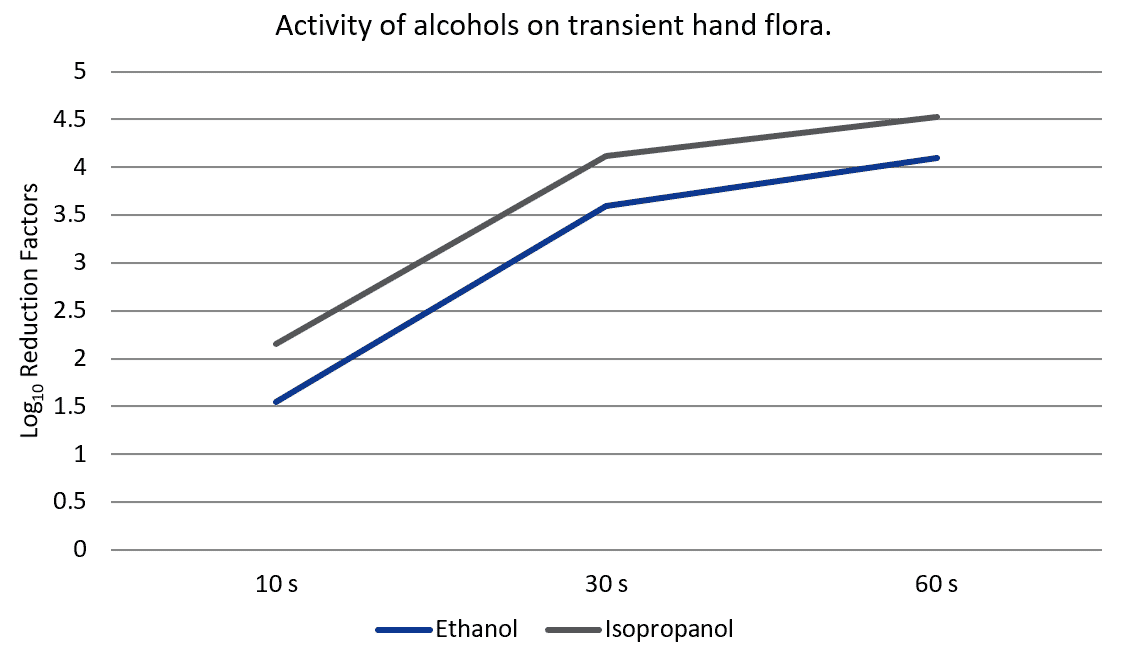

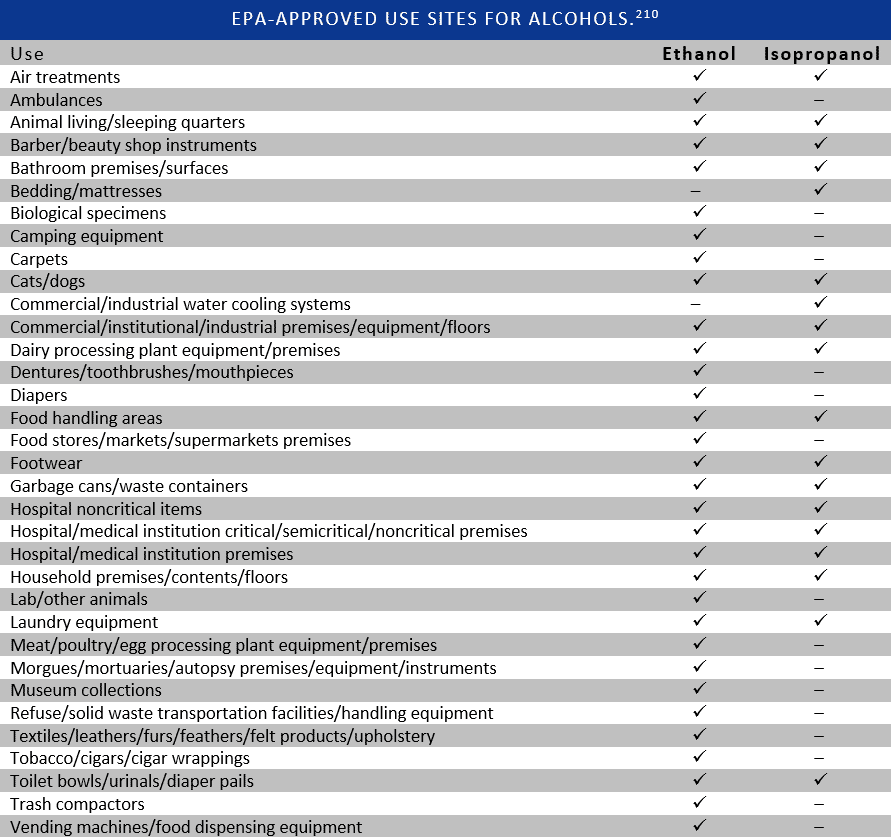

2 Comments
Dorotha Sensing
I am glad that I found this article well documented and very informative.
I want to share how I treated Yeast and Candida Infection, maybe
it will be useful to someone: https://bit.ly/3cq12iO
Thank you and keep going, you do a great job!!
ปั้มไลค์
Like!! I blog frequently and I really thank you for your content. The article has truly peaked my interest.